Microbial Culture Media For Quality Control Of Non-Sterile Products

lt;p>Using the correct media is critical to ensure microbiological quality. Explore a portfolio of culture media and substances for sample preparation, microbial enumeration tests, and tests for specified microorganisms.</p>

Validating Prefiltration Dirty-Hold Times - BioProcess International

Microbial Culture Media Merck Life Science Vietnam

Microbiology SpringerLink
Biologics Quality Control A Critical Component Of Development And Production
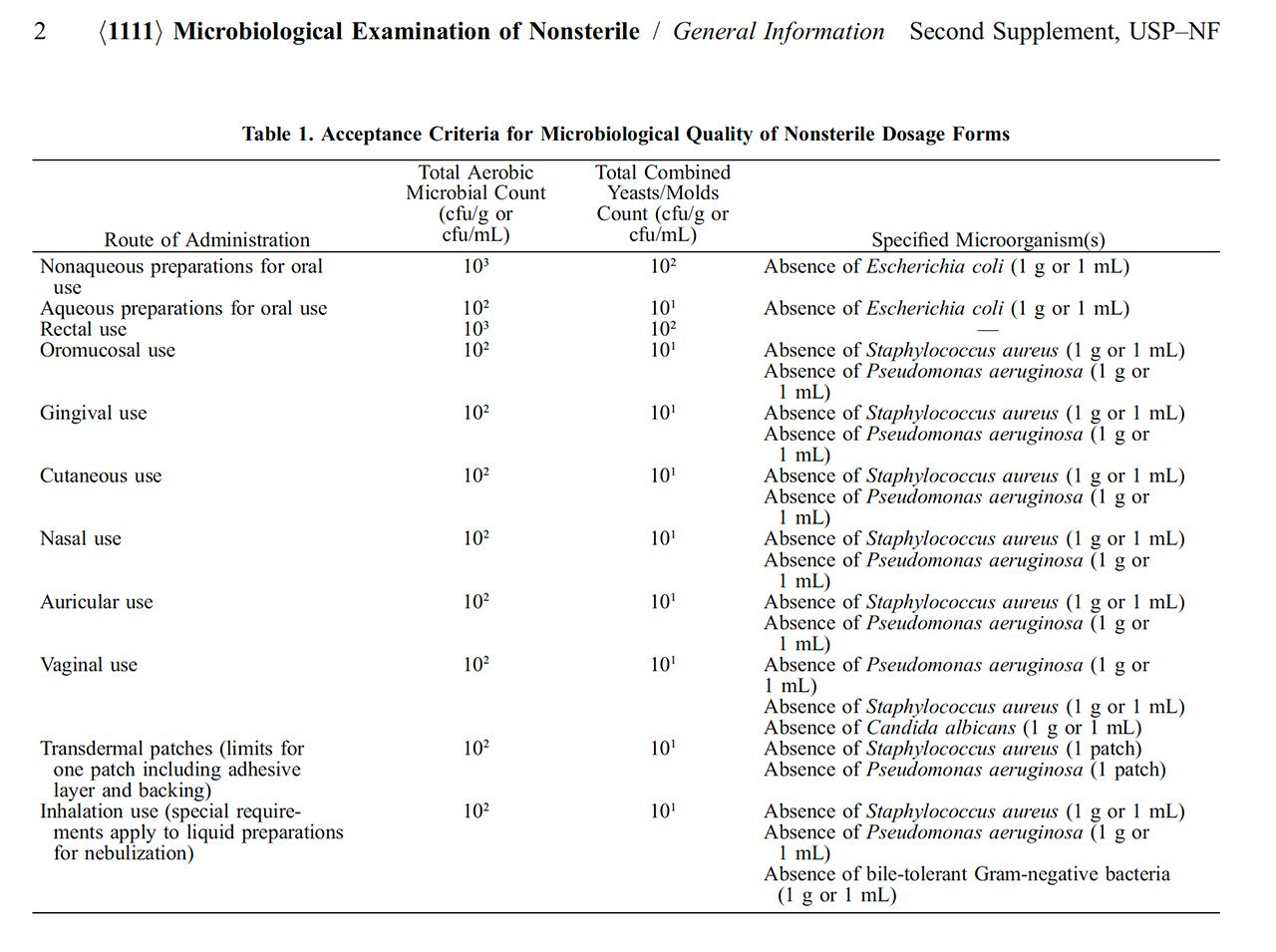
Refining Microbiological Control for Non-Sterile Products

Microbial Culture Media For Quality Control Of Non-Sterile Products
Microbial Culture Media- Definition, Types, Examples, Uses

Pharmaceutical Microbiology: Essentials for Quality Assurance and Quality Control: 9780081000229: Medicine & Health Science Books @
A Full Spectrum Of Compliant Ready-To-Use Mycoplasma Media With An Extended Shelf Life

Microbial Test Kit

Industrial Pharmaceutical Microbiology: Standards and Controls 6th Edi – euromedcommunications

The Steritest System — Benchmark Technology For Filtration-Based Sterility Testing

Making MS-Based Glycan Analysis Easier
Culture Media for Biological Indicators