What is the compressibility factor (Z) for 0.02 mole of a van der Waal

(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5
Filo Student Questions For CBSE , Grade 9 , Chemistry

Write the expression for the compressibility factor (Z) for one

Van Der Waals Equation - an overview

The compression factor (compressibility factor) one mole of a van

its mole fraction. Solution : P=KH⋅X⇒PCO2( g)=KH⋅X(CO2)⇒0.01=35×100..

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20
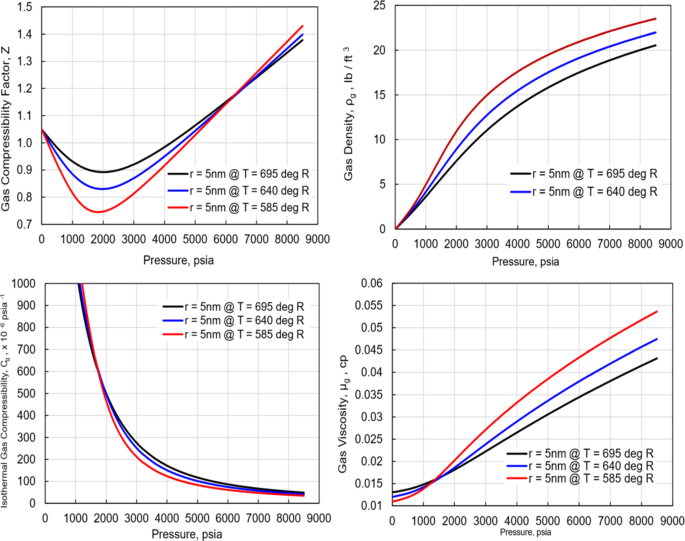
Investigation of the Properties of Hydrocarbon Natural Gases Under
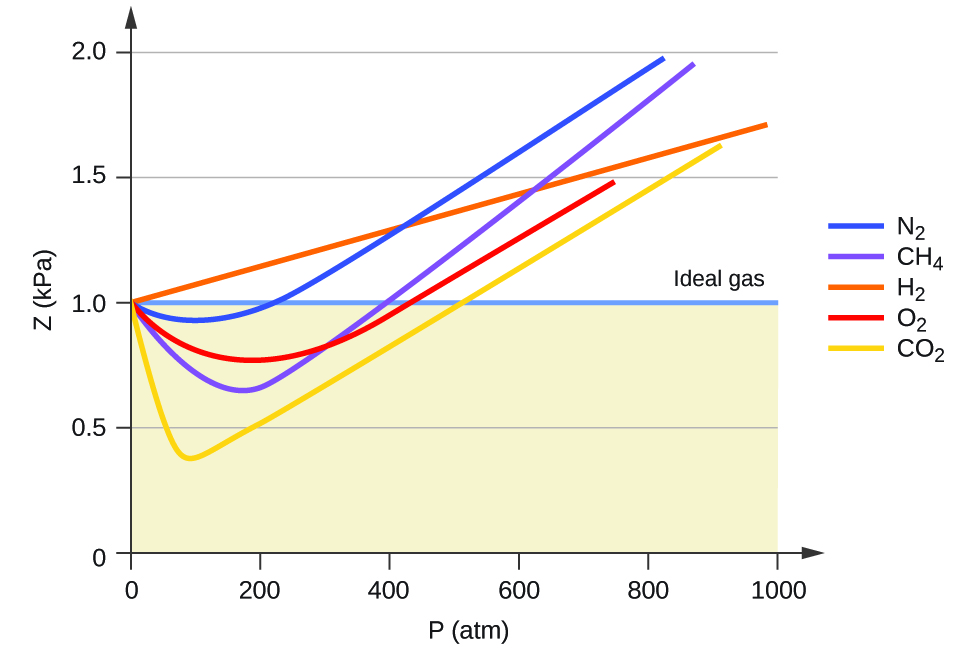
2.7: Non-Ideal Gas Behavior - Chemistry LibreTexts
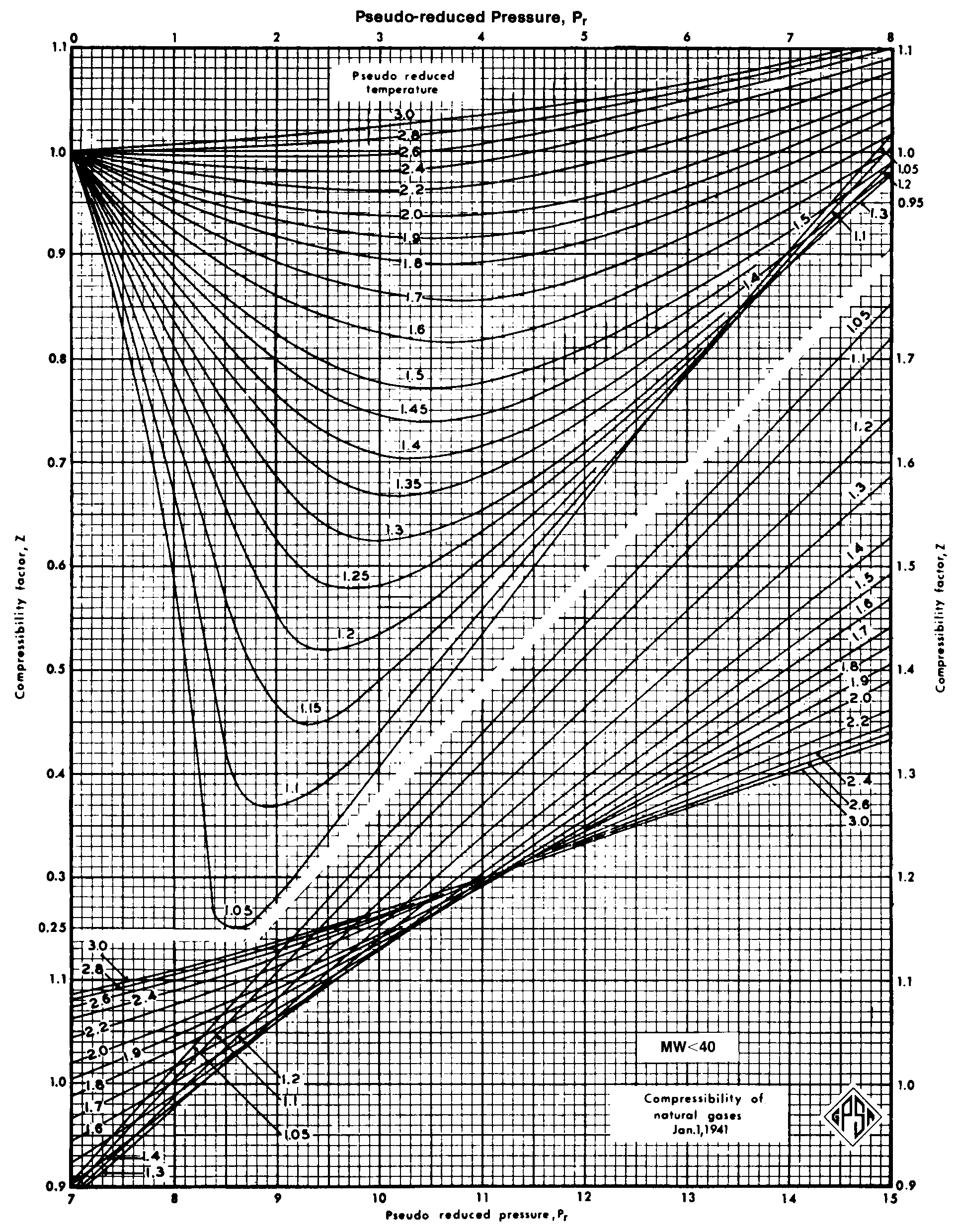
Determine Compressibility Factor, Z Factor - Engineering Units

DPP No. : 21 Total Marks : 40 Max. Time : 10mln Single choice Objective (..

Problem Set 2 Solutions

Answered: The van der Waals equation of state for…
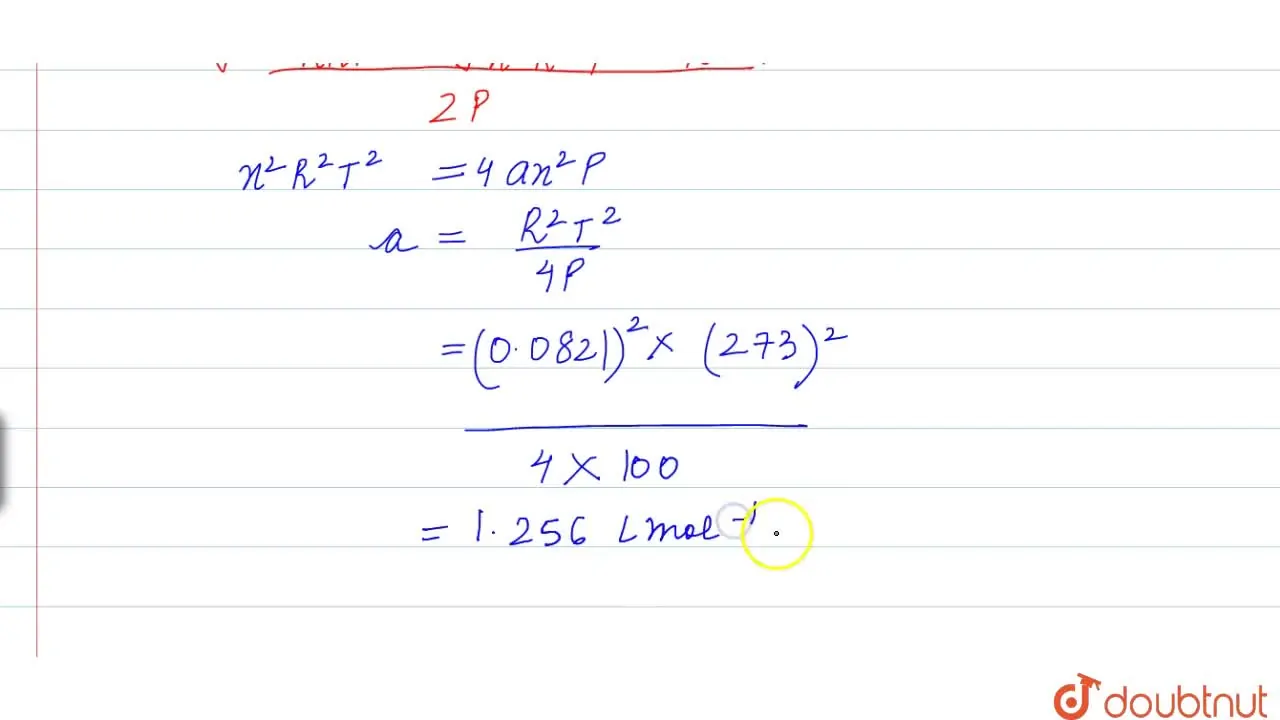
The compressibility factor for definite amount of van der Waals' gas a

One way of writing the equation of state for real gases is PV = RT [1+

plotting - How to plot Compressibility factor Z vs Pressure P