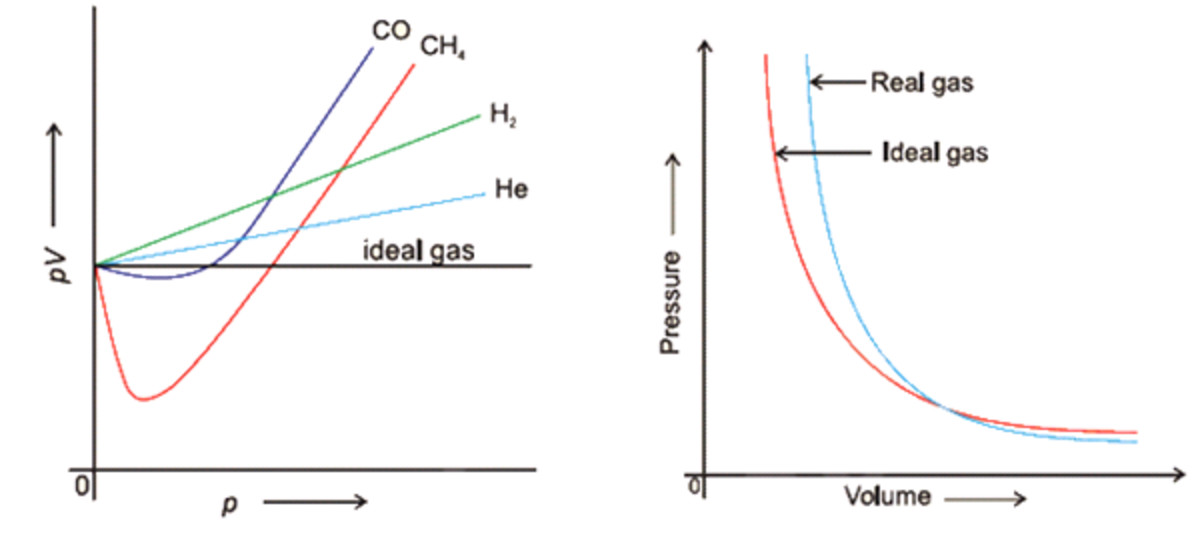
For H(2) gas, the compressibility factor,Z = PV //n RT is

For H(2) gas, the compressibility factor,Z = PV //n RT is
Real Gases - Chemistry, Class 11, States of Matter

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Compressibility factor - Wikipedia

e Compressibility factor (Z) for hydrogen WRT pressure and temperature
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1
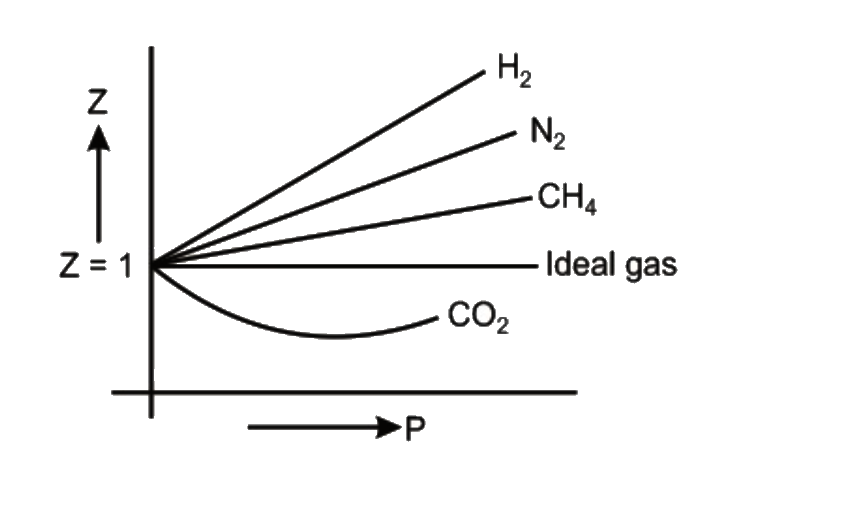
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Gas Compressibility - an overview

Gas Compressibility - an overview