Color change is only device modification. Is a new 510k required? - Medical Device Academy

This article explains the process for determining if a color change and other material changes require a new 510k prior to implementing the change.

US FDA Pre-Market Notification - 510(k)

FDA Guidance on 510(k) for Changes to Existing Devices
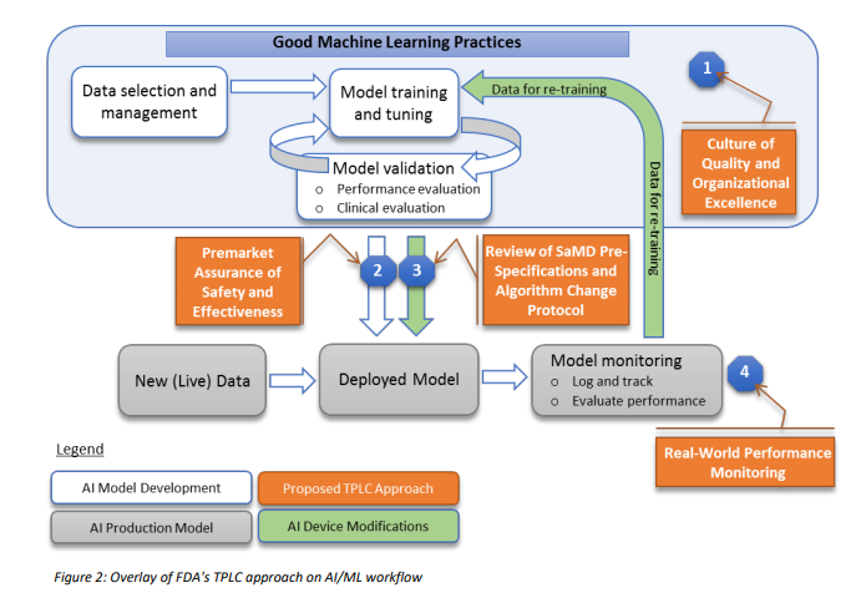
Digital Health Regulation: AI and Multiple Function Devices - Gsap

Medical Device Regulatory Training Requirements for Employees
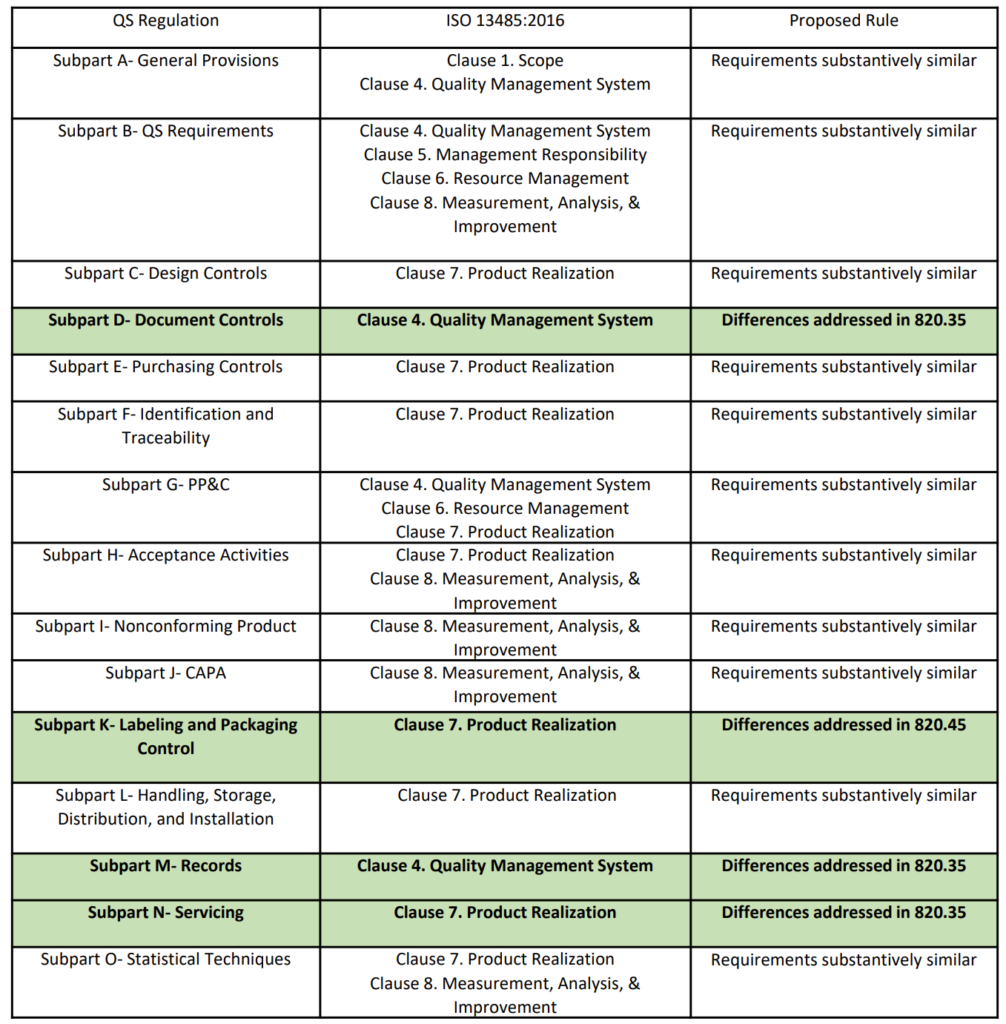
FDA
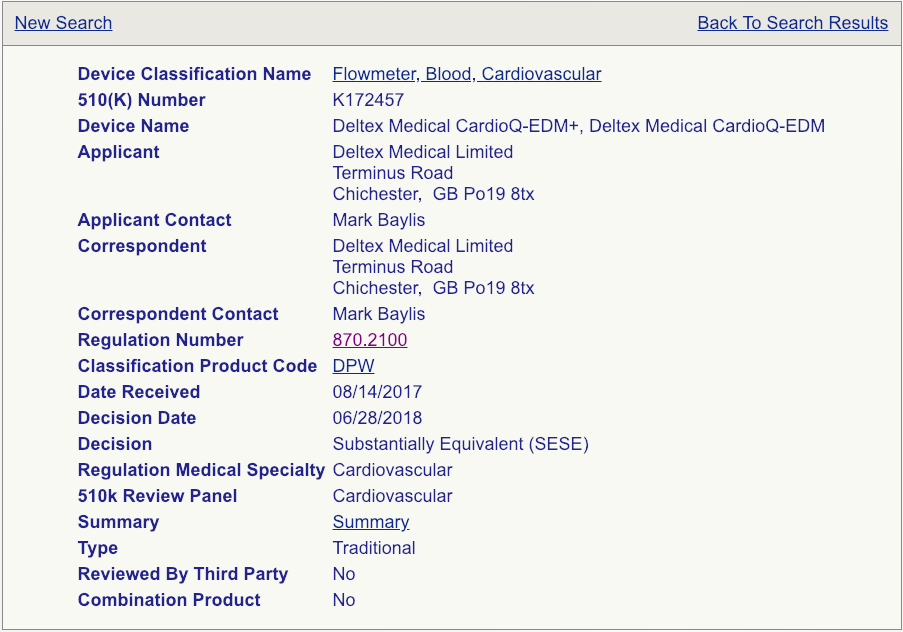
The FDA 510(k) Process: Setting the Stage for a Successful
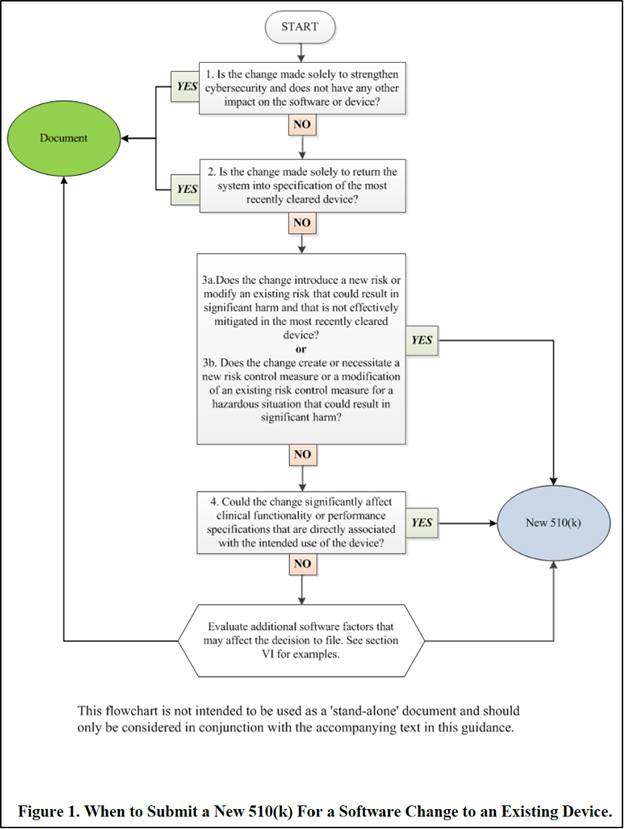
Medical Device Postmarket Change Controls FDA 510(k) Software
FDA
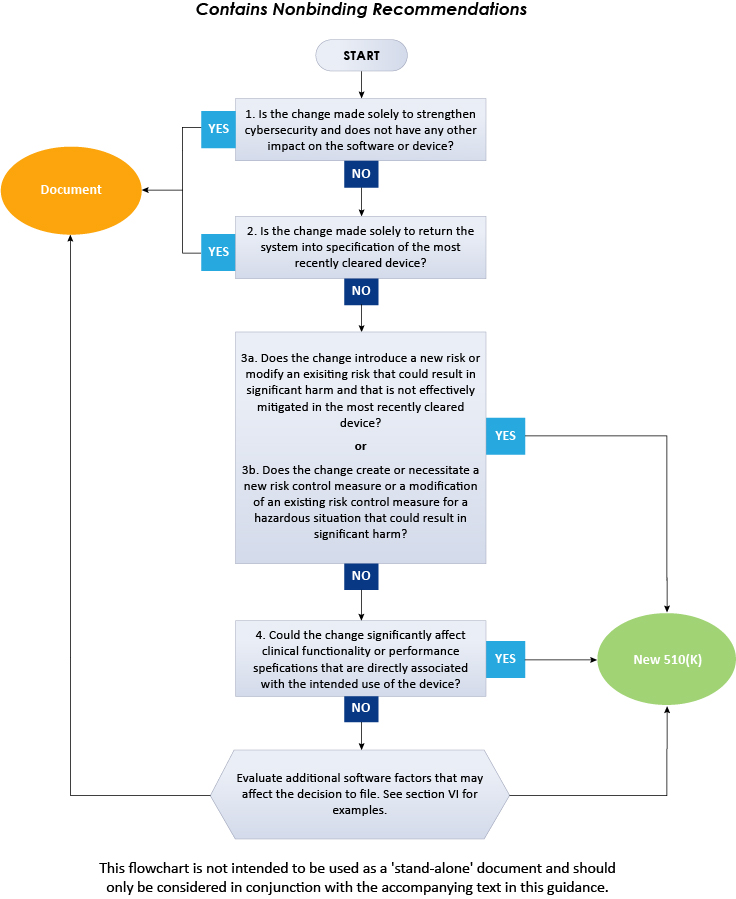
US FDA's Guidance on 510(k) Submission for a Software Change to an