Preparation of iron(IV) nitridoferrate Ca4FeN4 through azide
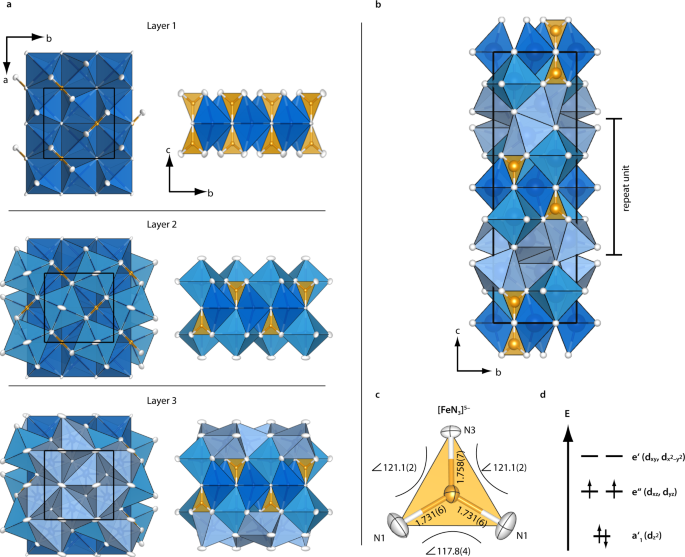
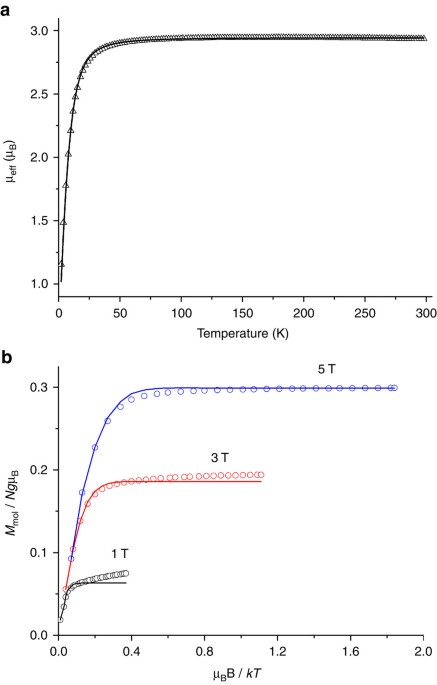
Indefinitely stable iron(IV) cage complexes formed in water by air oxidation
IRON(III) SULFATE N-HYDRATE, CAS#:15244-10-7
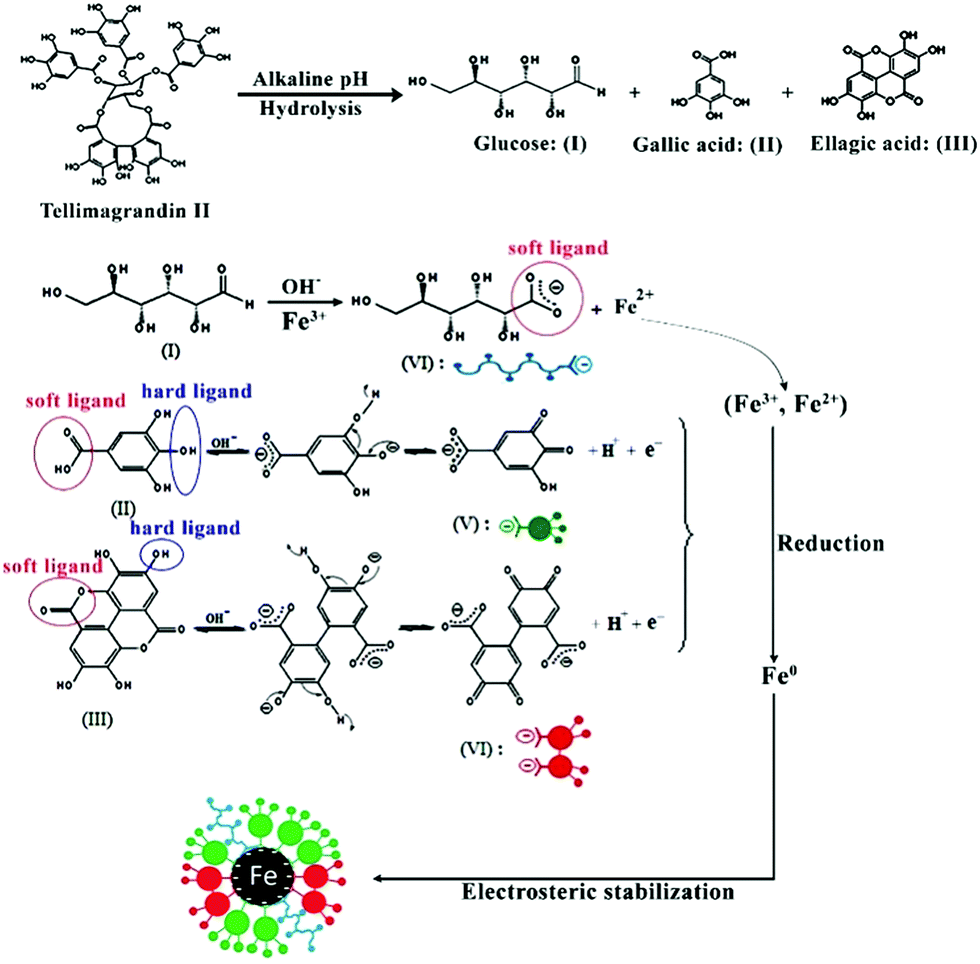
Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron-overl - RSC Advances (RSC Publishing) DOI:10.1039/C8RA04451A

Table 2 from XXXV. The Crystal Structure of Fe2P, Fe2N, Fe3N and
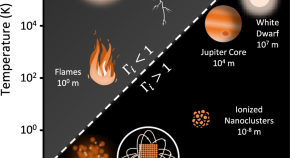
Research articles Nature Communications

Masato Goto's research works Kyoto University, Kyoto (Kyodai
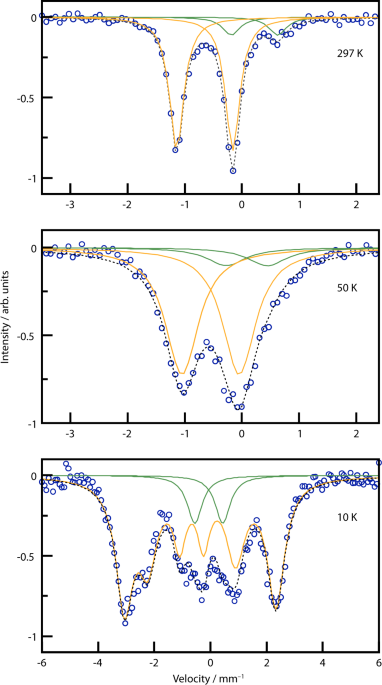
Preparation of iron(IV) nitridoferrate Ca4FeN4 through azide-mediated oxidation under high-pressure conditions

纳米人

Indefinitely stable iron(IV) cage complexes formed in water by air oxidation

Figure 8 from Synthesis of alkaline earth diazenides M(AE)N2 (M(AE) = Ca, Sr, Ba) by controlled thermal decomposition of azides under high pressure.