Solved An ideal gas initially at Pi, Vi, and Ti is taken
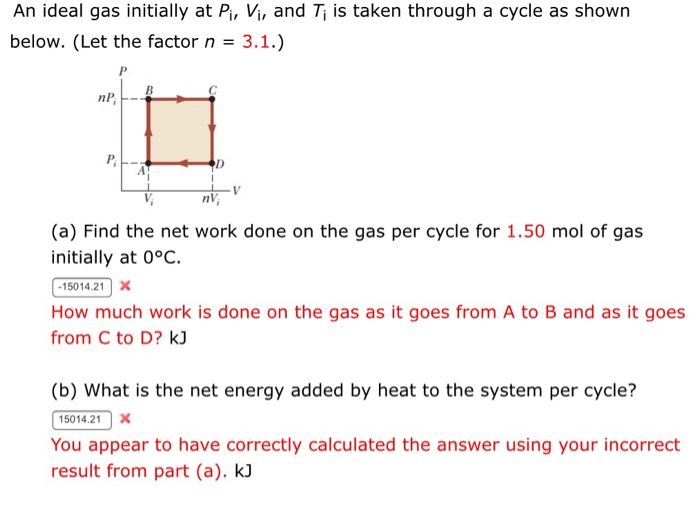

SOLVED: 3P; Pi A V 3V; A 1 mole of ideal gas initially at Pi-l Pa, Vi–5 m, and Ti= 0°C is taken through a cycle as shown in the above Figure.

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
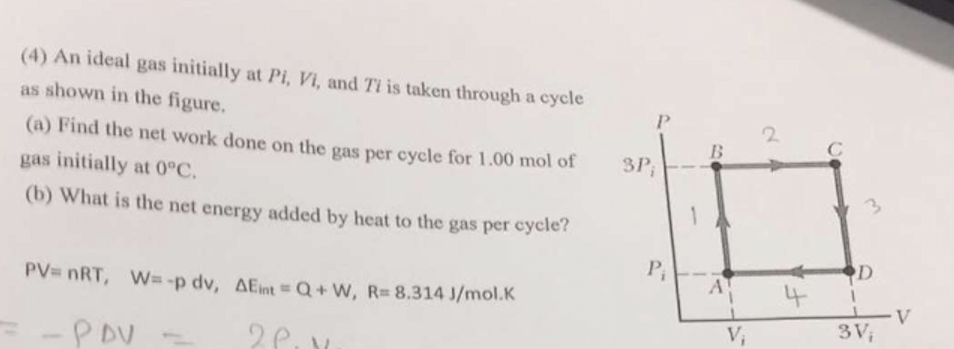
Solved (4) An ideal gas initially at Pi, Vi, and Ti is taken
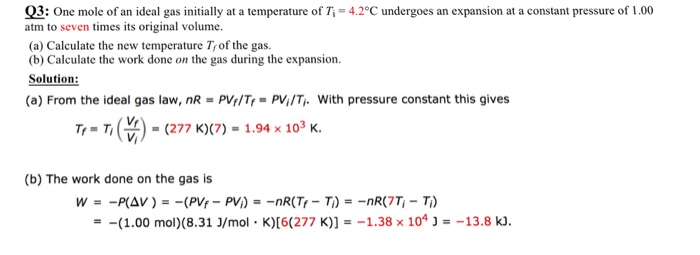
Solved For part b why is it 6 to multiple with 277 K ? I

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
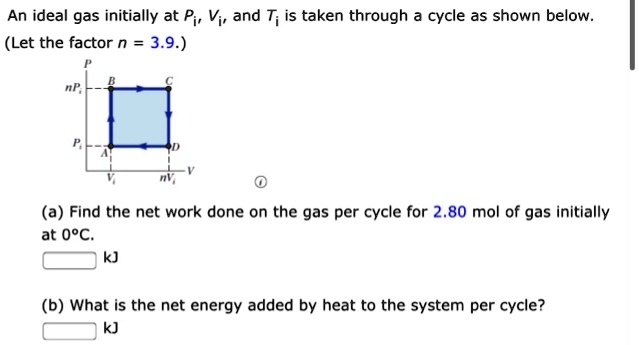
SOLVED: An ideal gas initially at Pi, Vi, and T; is taken through cycle as shown below (Let the factor n = 3.9.) (a) Find the net work done on the gas
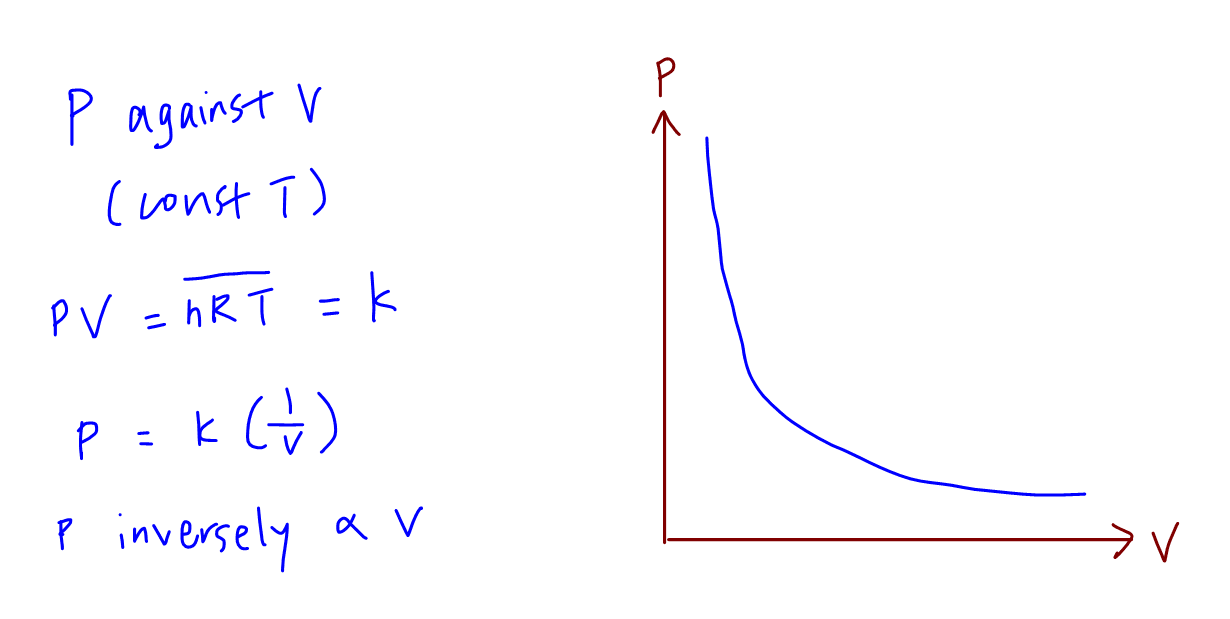
Ideal Gas Graph Sketching

Gas, Definition, State of Matter, Properties, Structure, & Facts
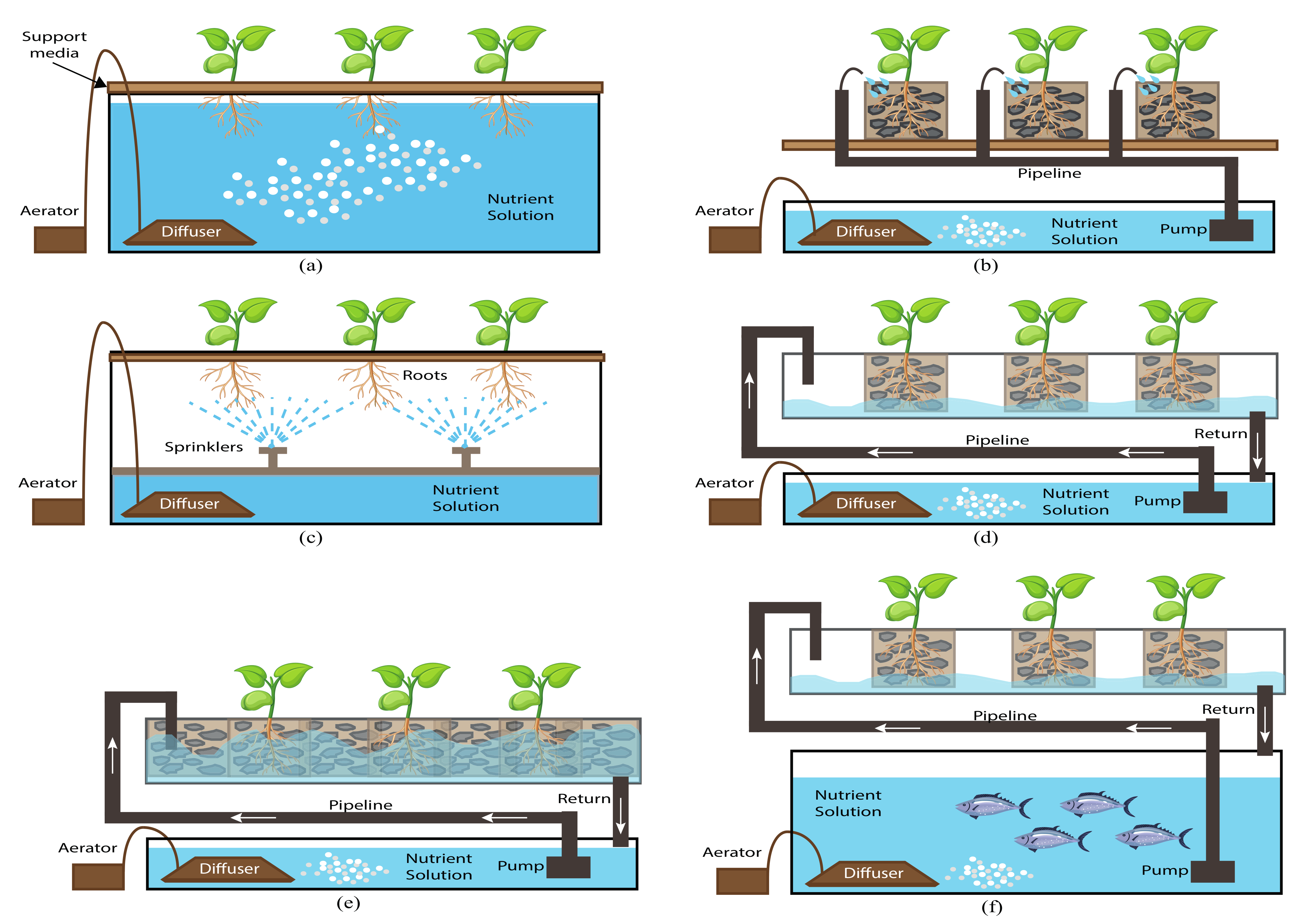
Agriculture, Free Full-Text
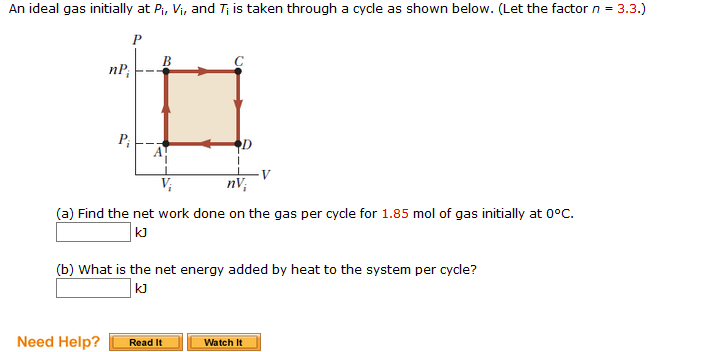
Solved An ideal gas initially at Pi, Vi, and Ti is taken

A 1.00 mol sample of monoatomic ideal gas is take through the cycle shown. At point A, the pressure, volume and temperature are P_i, V_i and T_i respectively. In terms of R

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of