At a high pressure, the compressibility factor (Z) of a real gas is us

At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z =(1-displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

Non-Ideal Gas Behavior Chemistry: Atoms First

Chemistry Desk: Effect of Pressure

Gas compressibility factor Z: Ideal gas vs Real gas

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Objectives_template

At a constant pressure, what should be the percentage increase in the

Solved] The compressibility factor for an ideal gas is

Non-Ideal Gas Behavior Chemistry: Atoms First
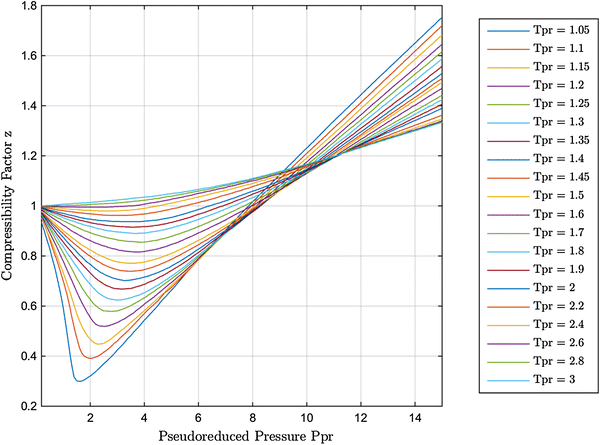
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms
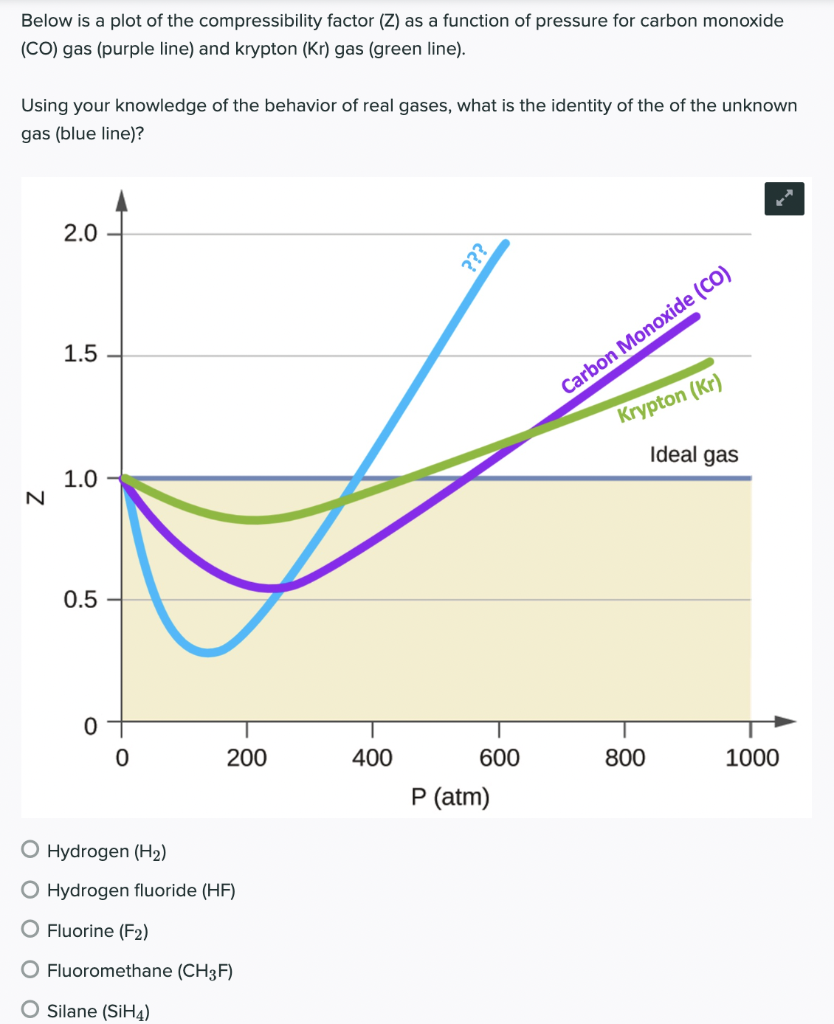
Solved Below is a plot of the compressibility factor (Z) as

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora