The compression factor (compressibility factor) for 1 mol of a van der

For 1 mol of a gas, the van der Waals equation is (P+(a)/(V(m)^(2)))(V(m)-b)=RT Ignoring b, we get (given volume of gas molecule is negligible) (P+(a)/(V(m)^(2)))V(m)=RT ltbgt or pV(m)+(a)/(V(m))=RT or (pV(m))/(RT)+(a)/(V(m)RT)=1 or Z=(pV(m))/(RT)=1-(a)/(V(m)RT) (i) It is given that Z=(pV(m))/(RT)=0.5implies V(m)=(0.5RT)/(P) With this, equation (i) becomes 0.5=1-(a)/((0.5RT//p)RT) or a=(0.5)((0.5RT)/(p))RT=0.25(R^(2)T^(2))/(p) Substiuting the given values, we get a=(0.25)[((0.082L atm K^(-1)mol^(-1))^(2)(273 K)^(2))/((100 atm))] =1.2528 L^(2) atm mol^(-2)

Which of the following equations represents the compressibility factor
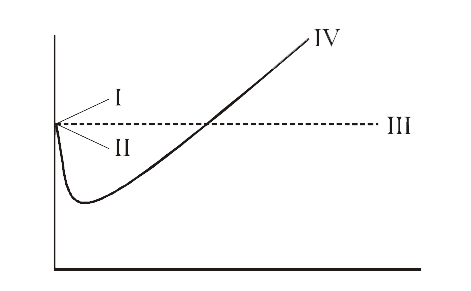
The compression factor (compressibility factor) for 1 mol of a van der
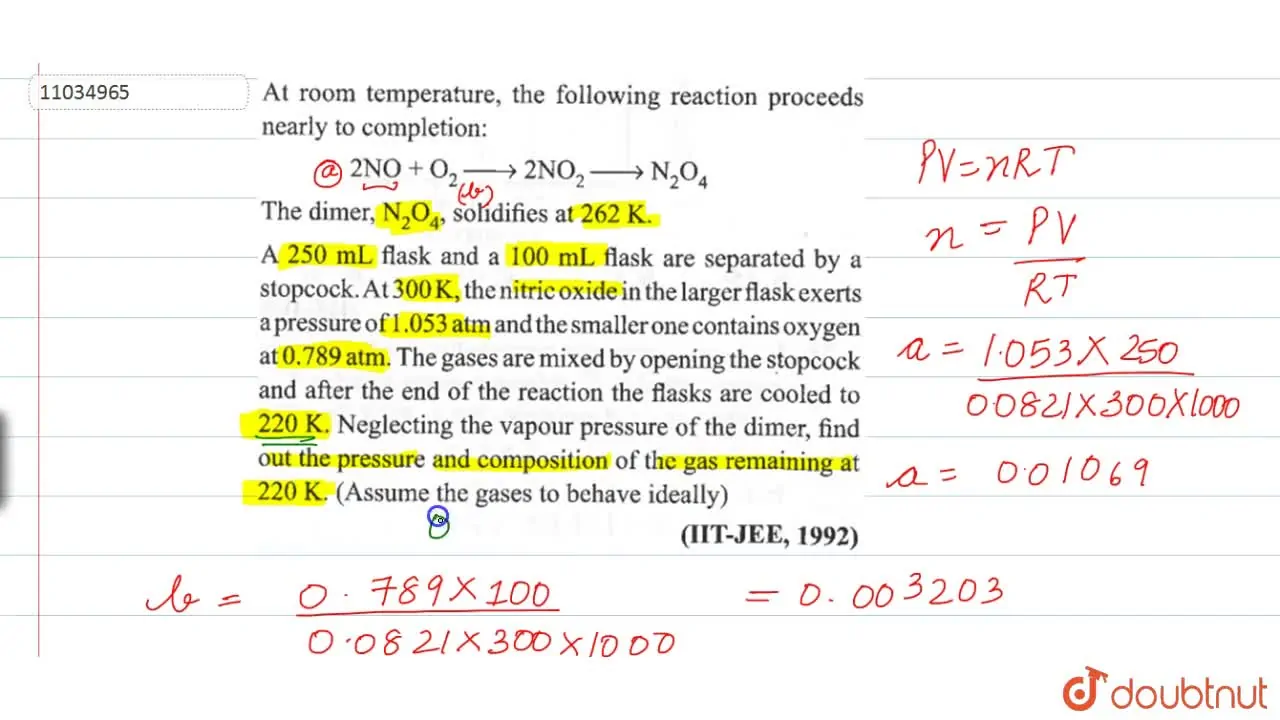
At room temperature, the following reaction proceeds nearly to complet
An LPG cylinder weighs 14.8 kg when empty. When full it weighs 29.0 kg

The compression factor (compressibility factor) for 1 mol of a van der
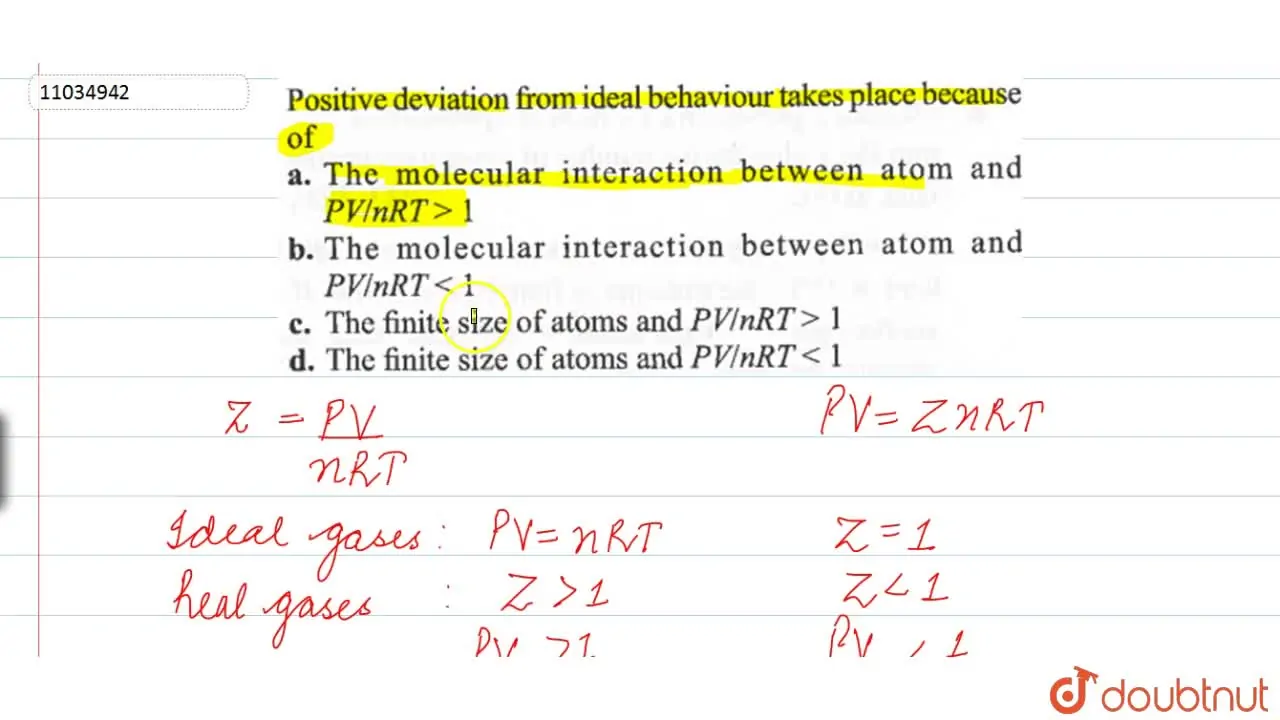
Finite size of atoms and PV // nRT gt 1
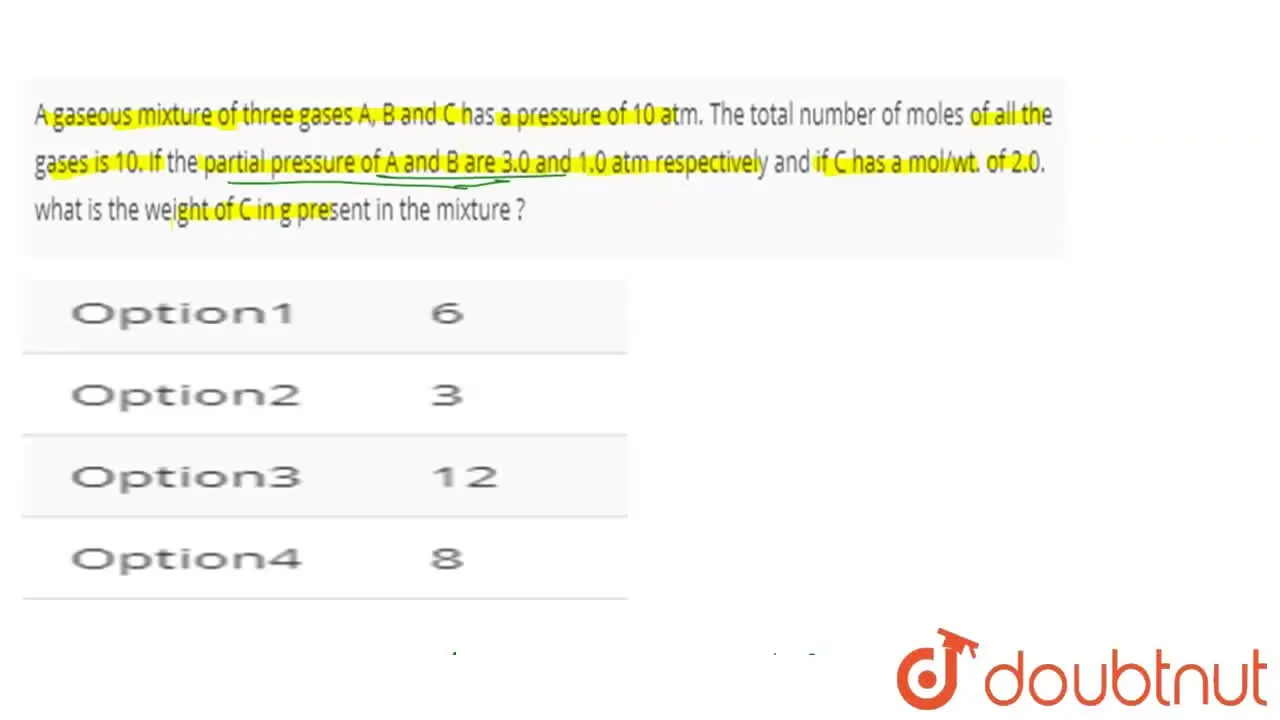
A gaseous mixture of three gases A, B and C has a pressure of 10 atm.

One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh
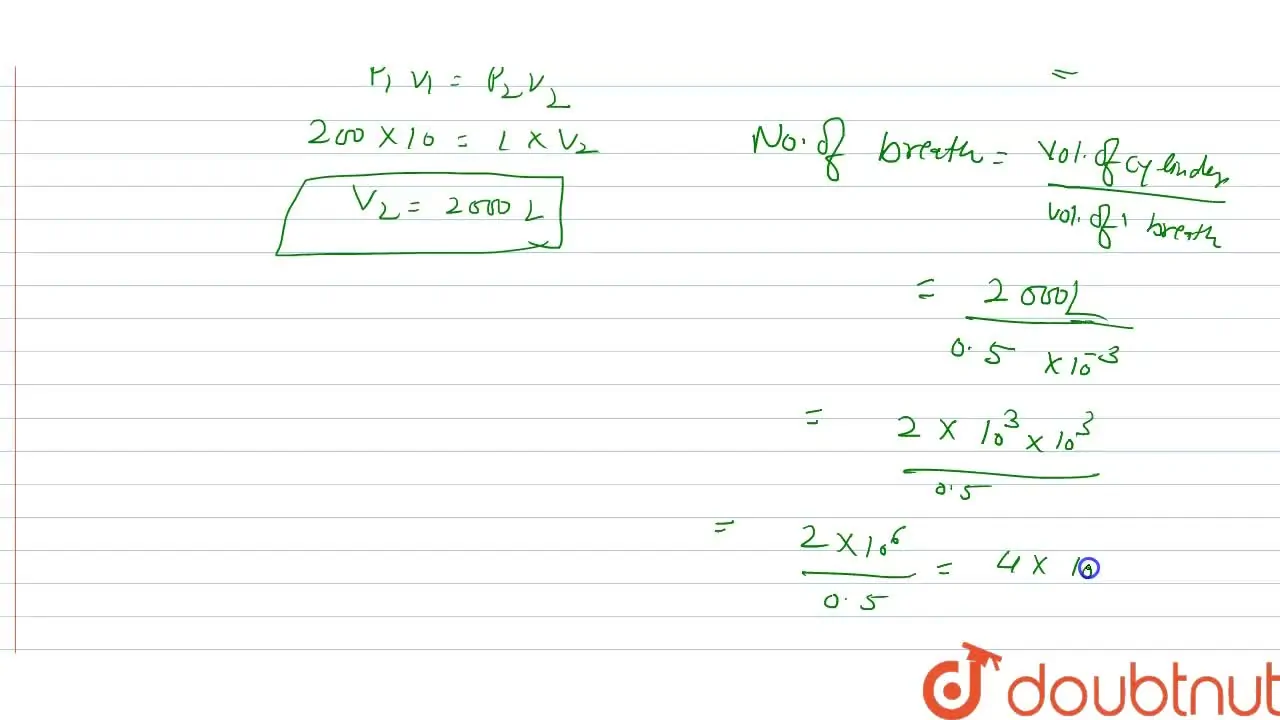
In a hospital, an oxygen cylinder holds 10 L of oxygen at 200 atm pres
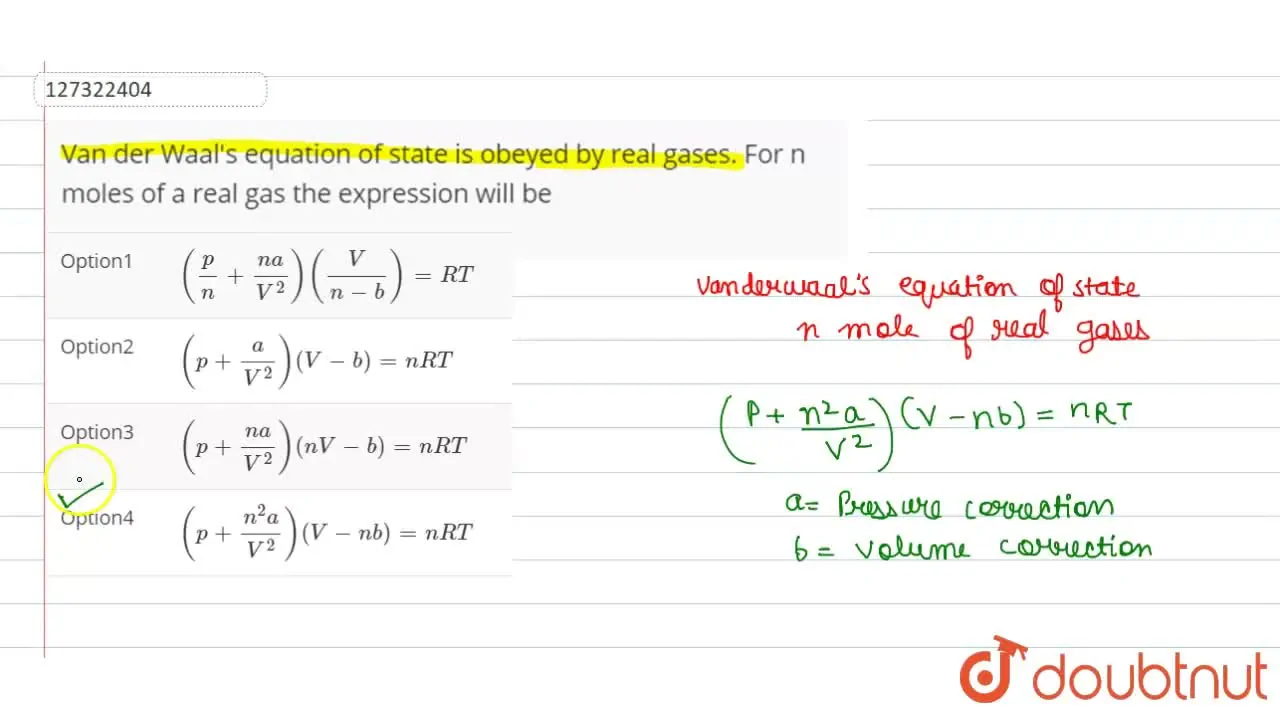
Van der Waal's equation of state is obeyed by real gases. For n moles
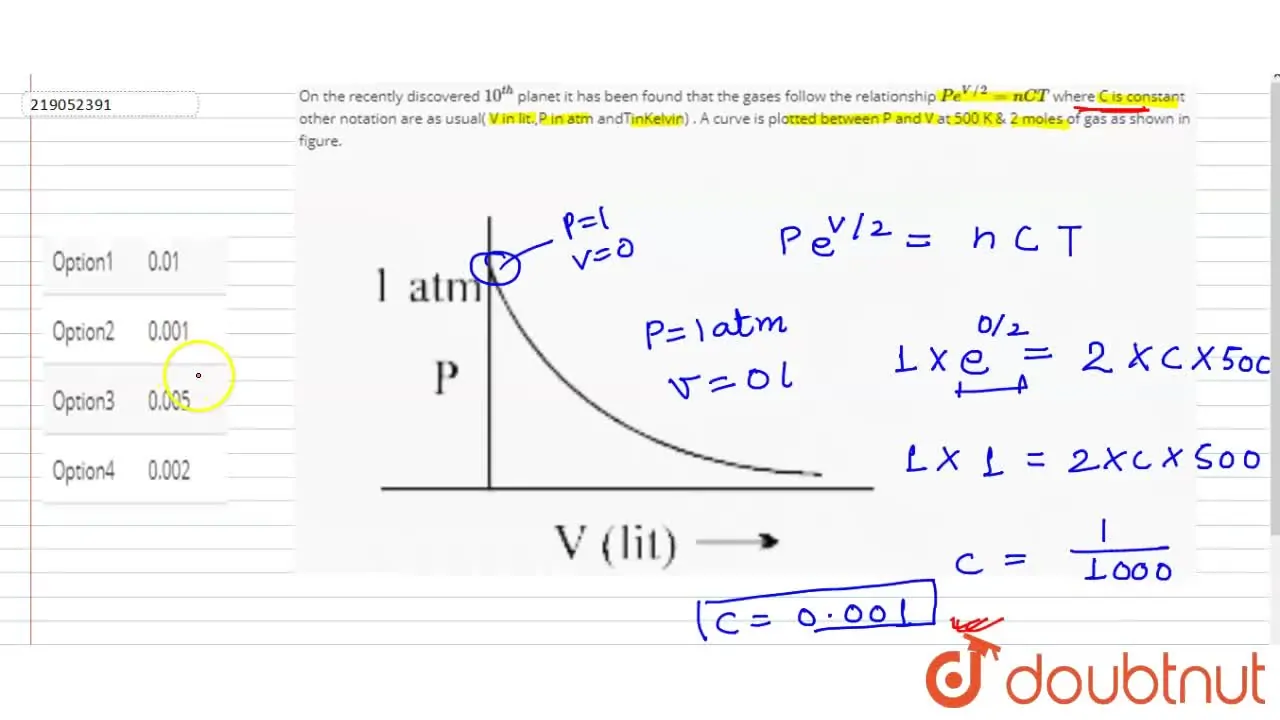
On the recently discovered 10^(th) planet it has been found that the
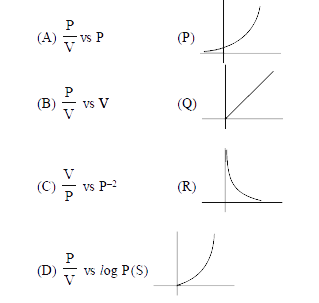
Match the description in Column I with graph provided in Column II. Fo