Molecular dynamic simulations reveal detailed spike-ACE2 interactions
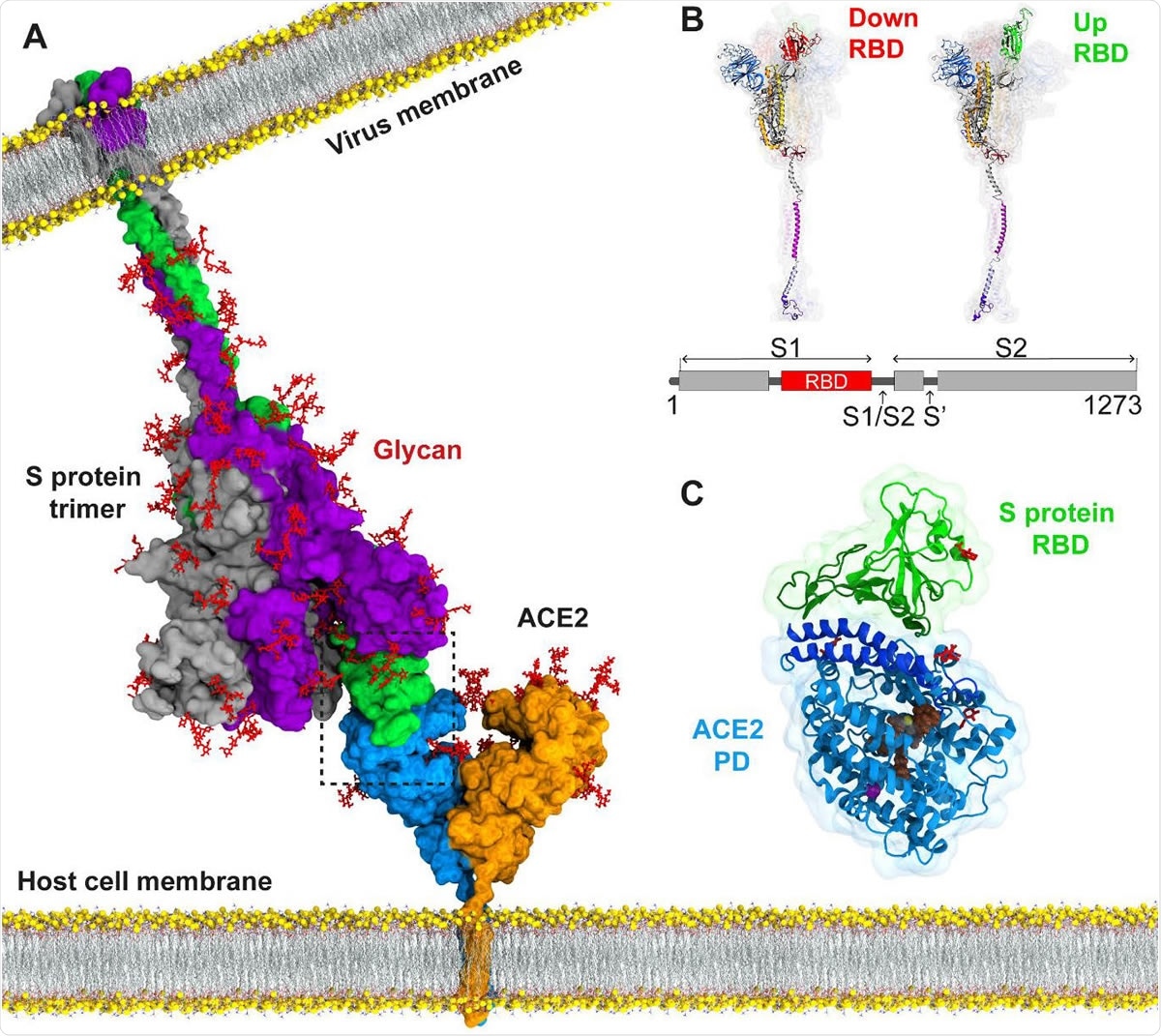
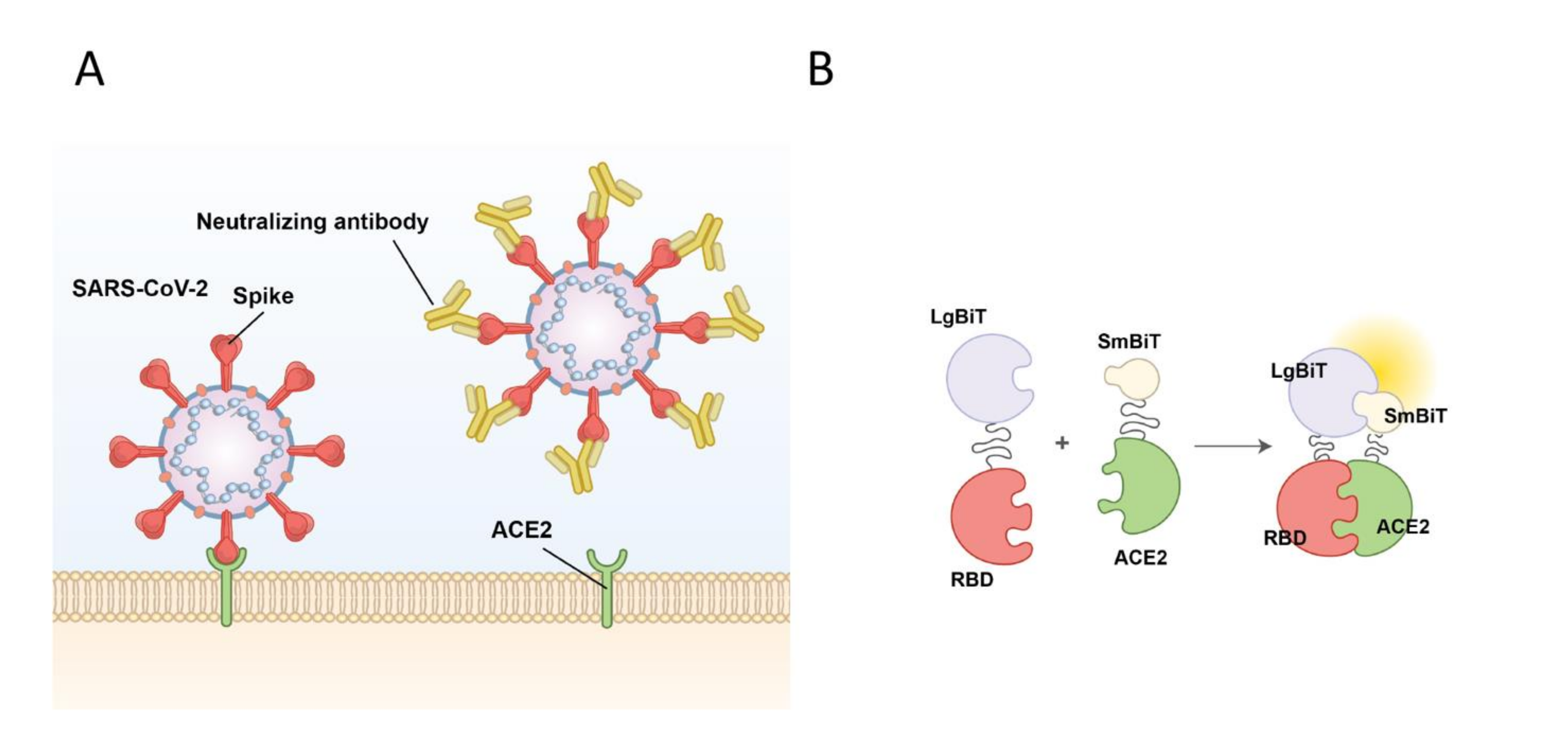
The current COVID-19 pandemic has spread throughout the world. Caused by a single-stranded RNA betacoronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is closely related to but much more infectious than the earlier highly pathogenic betacoronaviruses SARS and MERS-CoV, has impacted social, economic, and physical health to an unimaginable extent.

Computational simulations reveal the binding dynamics between human ACE2 and the receptor binding domain of SARS-CoV-2 spike protein

Molecular dynamic simulation suggests stronger interaction of Omicron-spike with ACE2 than wild but weaker than Delta SARS-CoV-2 can be blocked by engineered S1-RBD fraction
Molecular Interaction And Inhibition Of SARS-CoV-2 Binding, 54% OFF

Cells, Free Full-Text
Molecular Interaction And Inhibition Of SARS-CoV-2 Binding, 54% OFF

Computational biophysical characterization of the SARS-CoV-2 spike protein binding with the ACE2 receptor and implications for infectivity - Computational and Structural Biotechnology Journal
Unraveling SARS-Cov-2 spike protein conformational dynamics under the influence of electric fields - NHR4CES

Mutational landscape and in silico structure models of SARS-CoV-2 spike receptor binding domain reveal key molecular determinants for virus-host interaction, BMC Molecular and Cell Biology
PDF) Dynamics of the ACE2–SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms
A molecular dynamics simulation study of the ACE2 receptor with screened natural inhibitors to identify novel drug candidate against COVID-19 [PeerJ]

In silico comparison of SARS-CoV-2 spike protein-ACE2 binding affinities across species and implications for virus origin
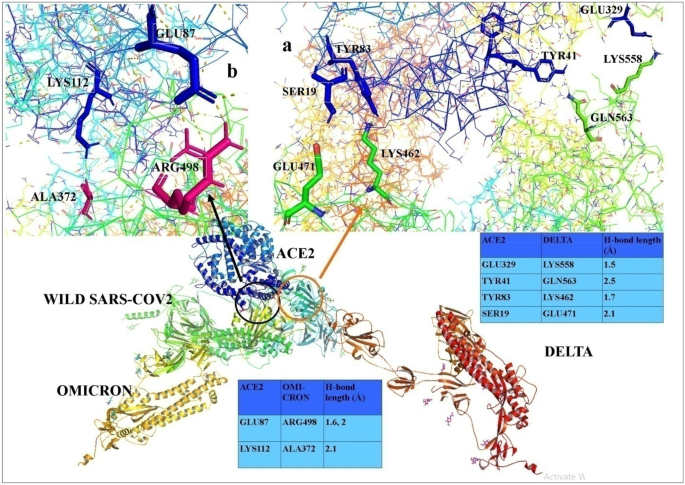
Molecular dynamic simulation suggests stronger interaction of Omicron-spike with ACE2 than wild but weaker than Delta SARS-CoV-2 can be blocked by engineered S1-RBD fraction

Comparison between experimental and modeled SARS-CoV-2 Spike-Ace2
Molecular Interaction And Inhibition Of SARS-CoV-2 Binding, 54% OFF
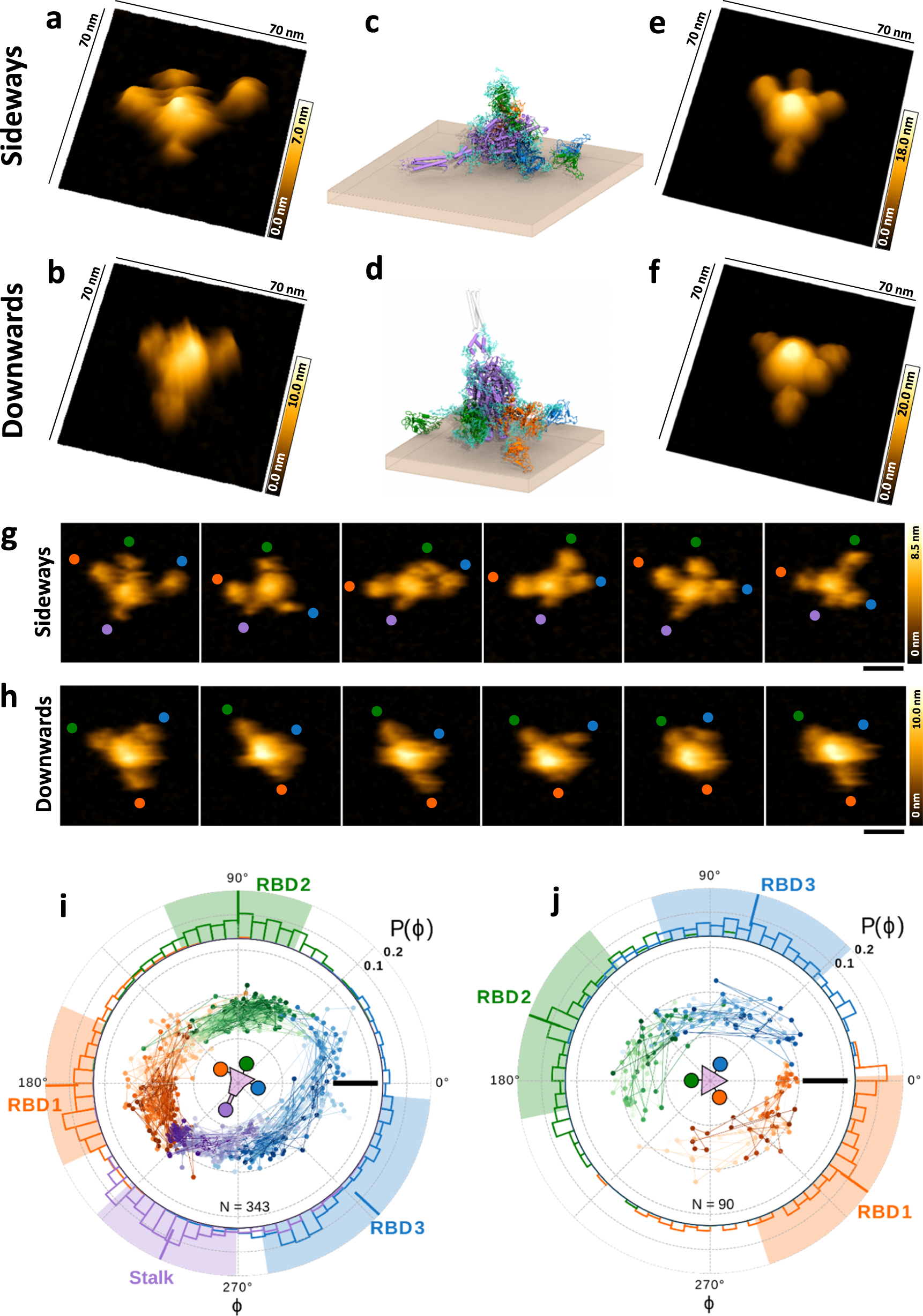
Force-tuned avidity of spike variant-ACE2 interactions viewed on the single-molecule level