The entropy change for the conversion of 36 g water to vapour at
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1

Calculate the entropy change in JK 1 mol 1 for vaporisation of liquid water to steam at 100∘ C. Given that heat of vaporisation is 40.8 kJ mol 1A. 109.38B. 100.38C. 120.38D. 129.38
8.What is the entropy change when one mole of ice is converted into water at 0 degree Celsius? (the entropy change for the conversion of ice to liquid water is 6.0 kJ
36 g of water at 30°C are converted into steam at 250°C at constant atmospheric pressure. - Sarthaks eConnect

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 K is: (Specific heat of water liquid and water vapour are 4.2 kJ K-kg
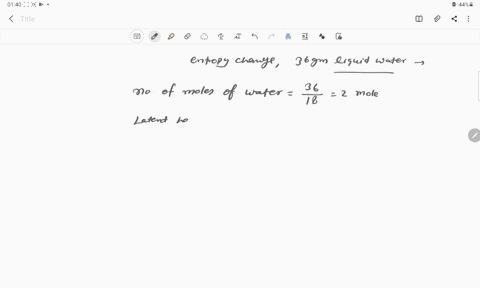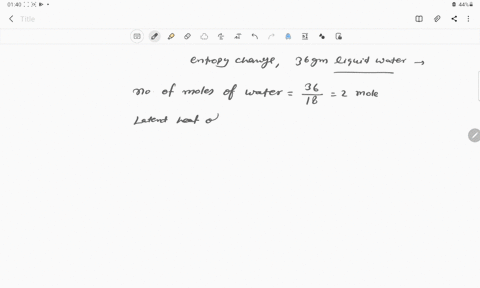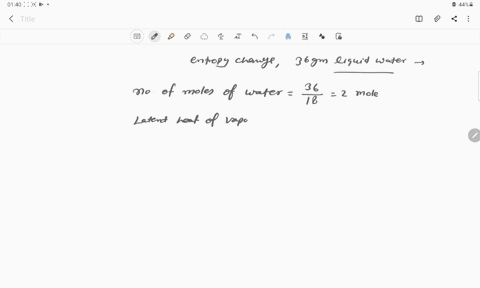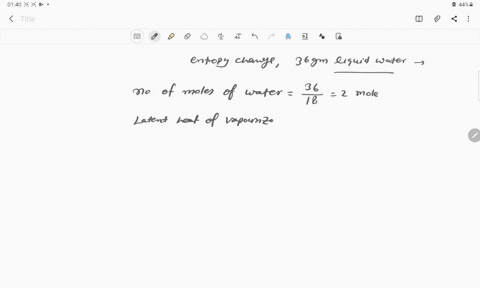
⏩SOLVED:Calculate the entropy change for the conversion of…
The latent heat of vapourisation of water at 100 Celcius is 540 cal/g . Calculate the entropy increase when one mole of water at 100 Celcius is evaporated.

The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 38

JEE Main Previous Year Questions (2016- 2023): Thermodynamics - 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download

The concept of dynamic evaporation enabled by reconfigurable Fe3O4@G

The entropy change when 36g of water evaporates at 373 K is :- (DeltaH

At 373 K, the entropy change for the transition of liquid water to ste

calculate the entropy change involved in conversion of one mole (18g) of solid ice at 273 K of liquid water - Chemistry - Thermodynamics - 9709217