The Cottrell Experiment and Diffusion Limitation 3/3 - Electrochemical Double Layer - PalmSens

In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
(PDF) Finite Heterogeneous Rate Constants for the Electrochemical

Alternative representation of the Cottrell diffusion according to
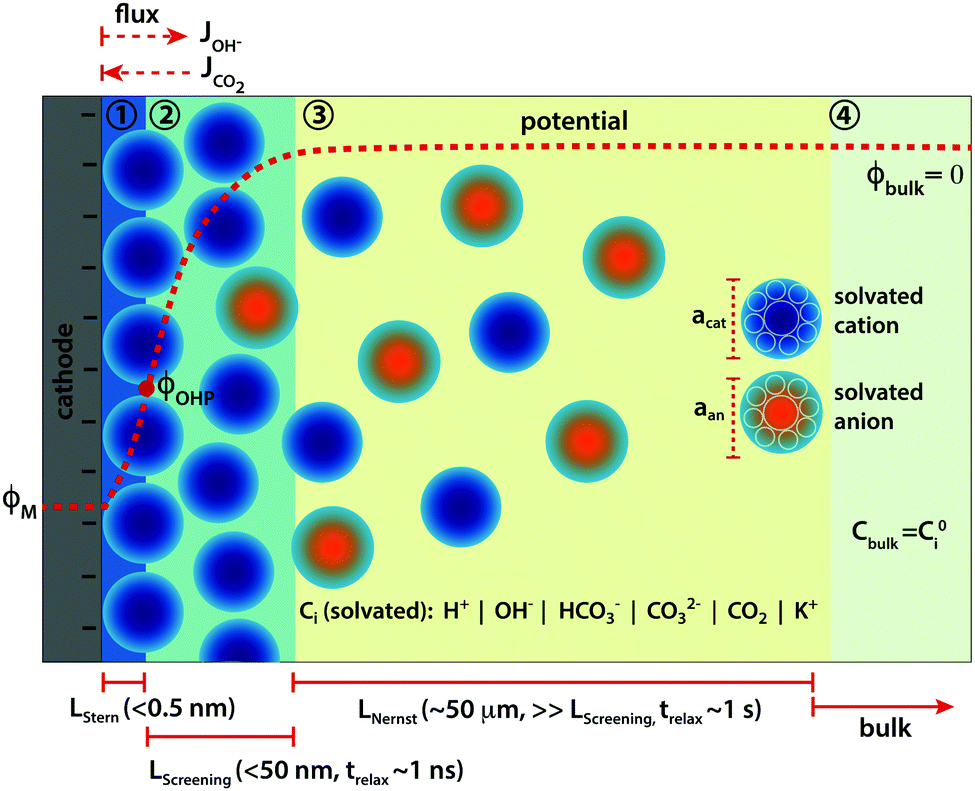
Modeling the electrical double layer to understand the reaction
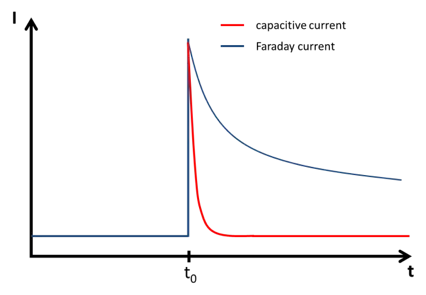
Capacitive Current - PalmSens
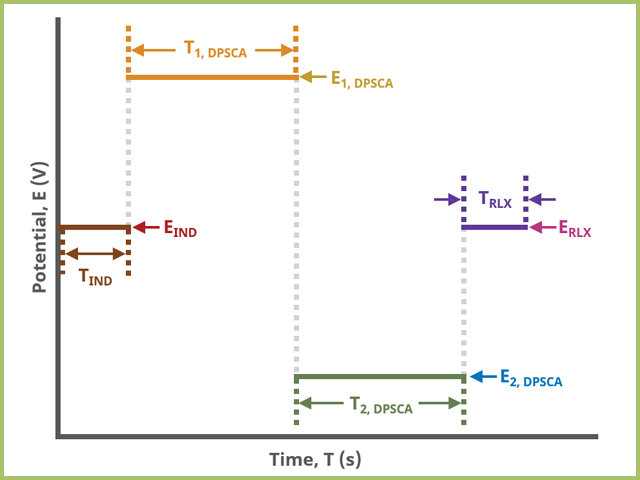
Double Step Chronoamperometry (DPSCA) – Pine Research
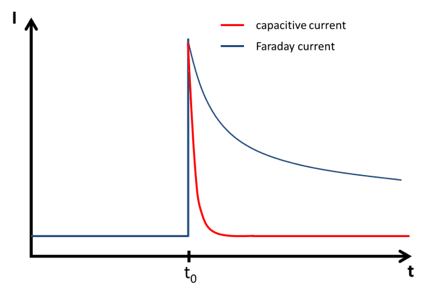
Capacitive Current - PalmSens

Electrochemical Impedance Spectroscopy (EIS) - PalmSens

The Cottrell Experiment and Diffusion Limitation 2/3 - The

Cottrell's equation revisited: an intuitive, but unreliable, novel

Electrochemical Double Layer - an overview

PDF) Bioinspired Chemically Modified Electrodes for Electroanalysis