For a given gas, a graph is shown between compressibility factor

By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

gas laws - Compressible Factor - Chemistry Stack Exchange

physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange
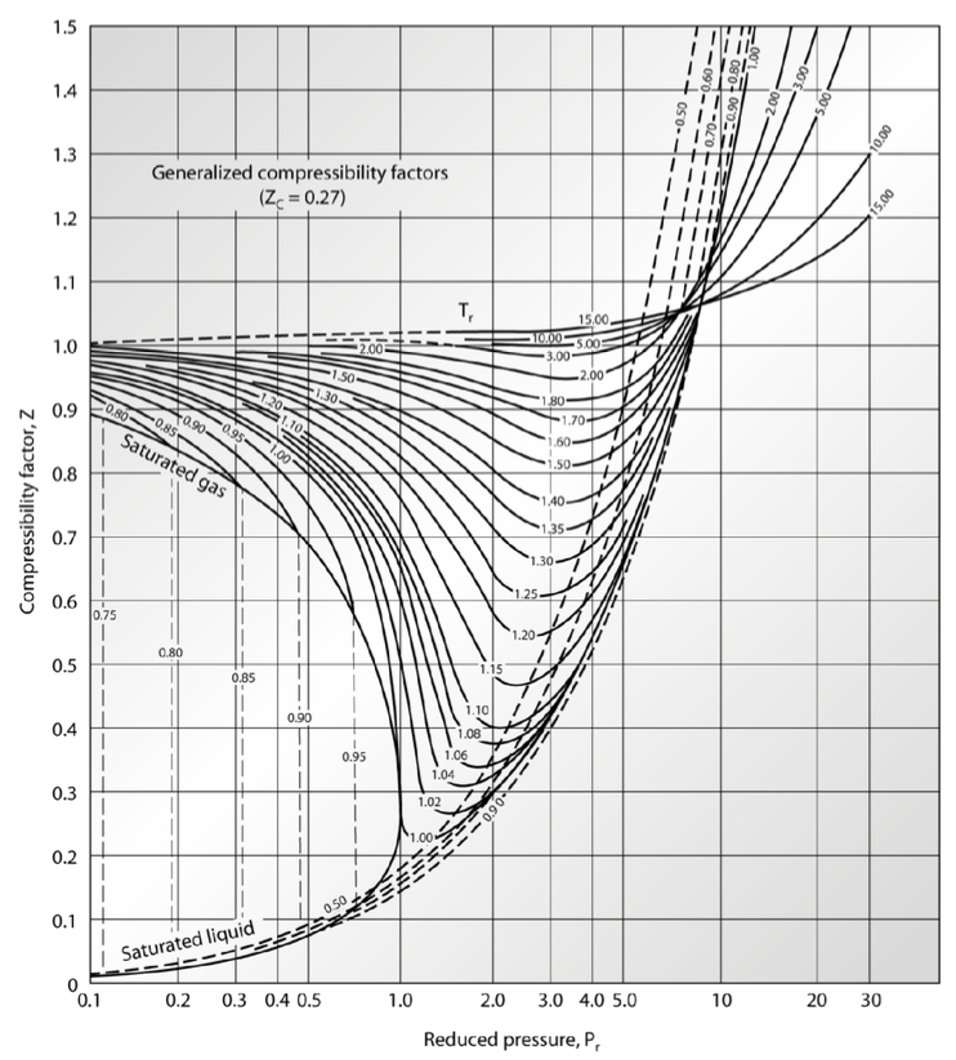
Qin Lab - thermal data

Chemistry Desk: Effect of Pressure

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P
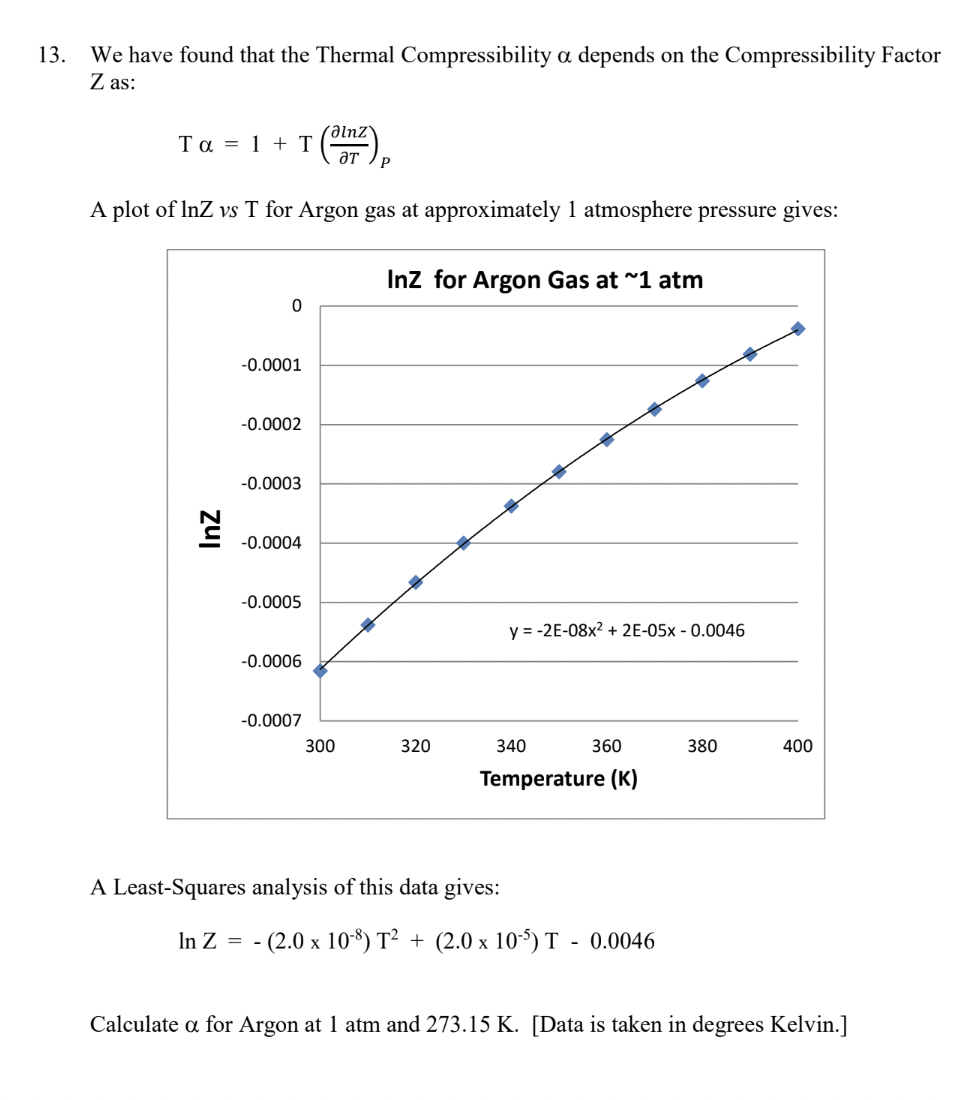
Solved 13. We have found that the Thermal Compressibility α

The following graph is plotted between compressibility factor Z versus pressure of a gas at different temperatures.Which of the following statements is /are correct?
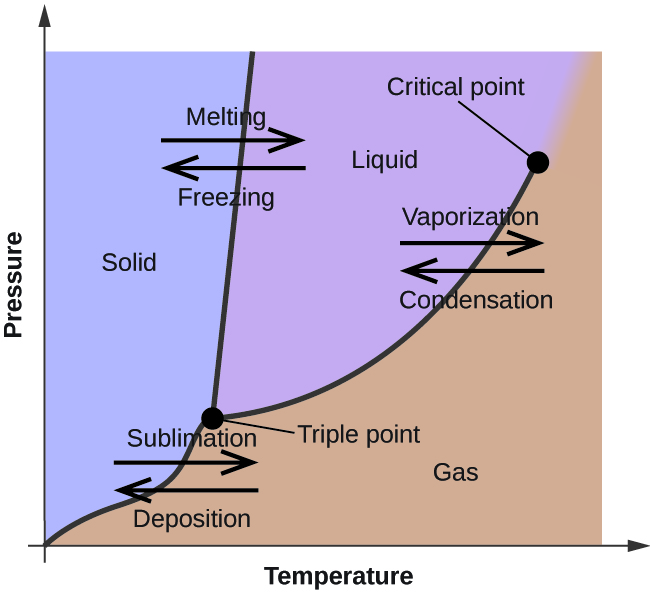
Phase Diagrams Chemistry for Majors

Reading Compressibility Factor Charts
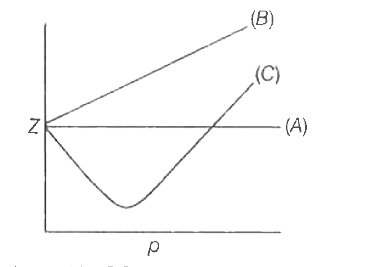
Telugu] The variation of compressibility factor (Z) with pressure (p

Variation of both compressibility factor and viscosity of gas with