If `Z` is a compressibility factor, van der Waals' equation at low

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
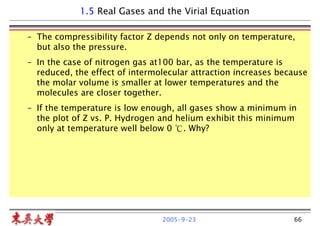
Real Gases and the Virial Equation

Compressibility Factor of Gas Overview, Equation & Chart
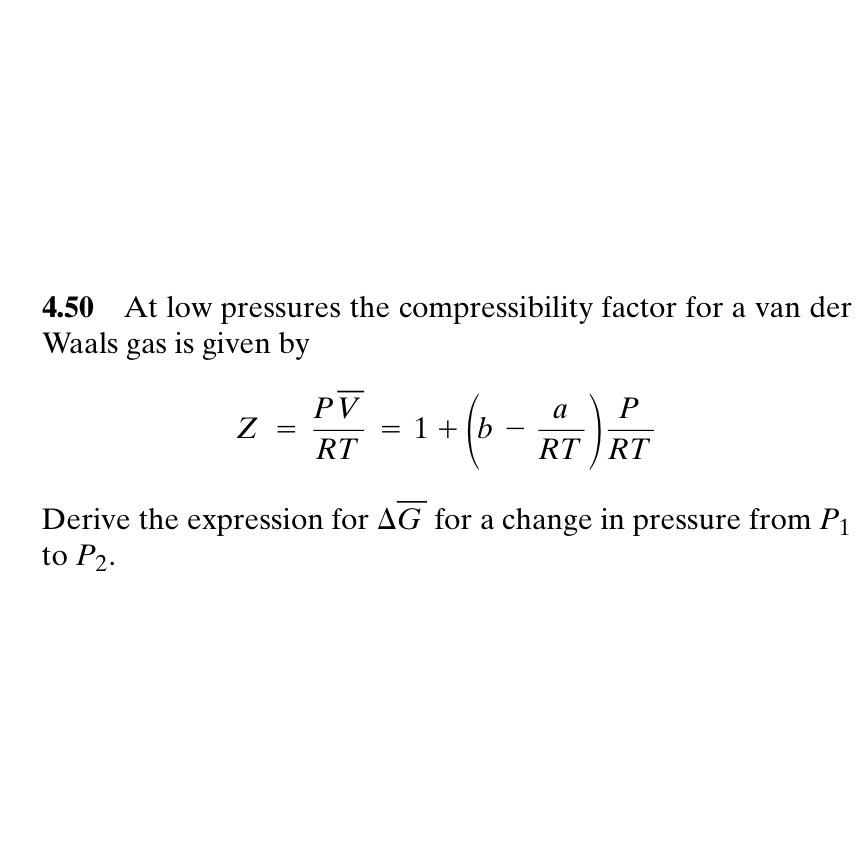
Solved 4.50 At low pressures the compressibility factor for

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

The compressiblity factor a gas obeying van der Waals' equation of

SOLVED: The fugacity of a van der Waals gas can be determined

If Z is a compressibility factor, van der Waals' equation at low

Given Vapour pressure of H 2 O at 300 K is 3170 Pa R 8314 JK 1 mol
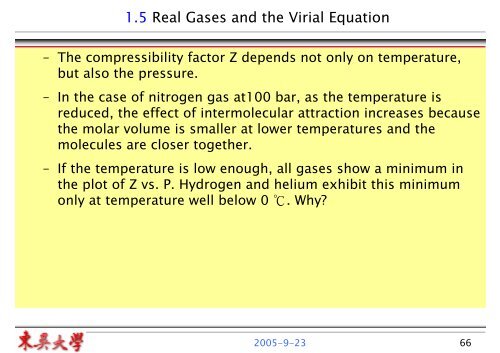
1.5 Real Gases and the Virial Equation - Mail