Compressibility factor (Z) for a van der Waals real gas at critical point is

Share your videos with friends, family and the world

Determine Compressibility of Gases
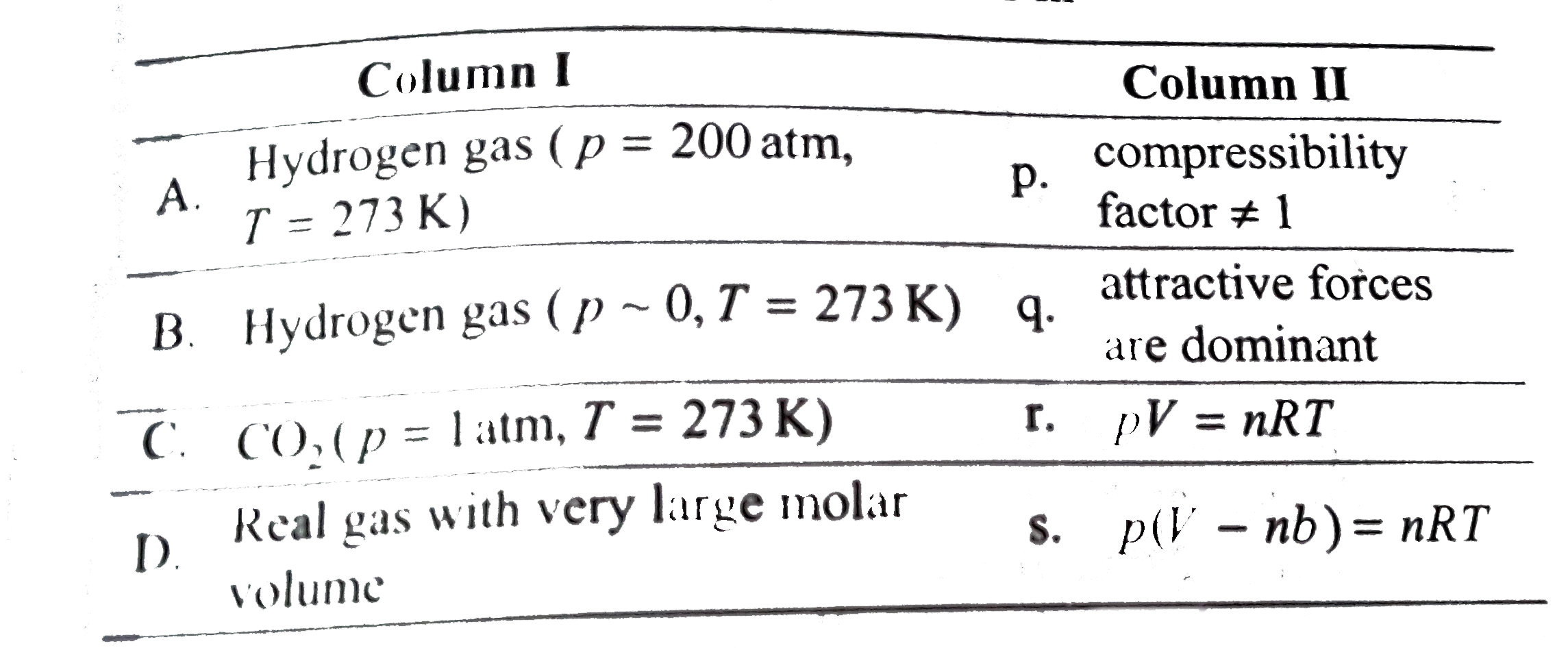
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

Solved Problem 1. 15 points Find the compressibility factor

Under critical states of a gas for one mole of a gas, compressibility
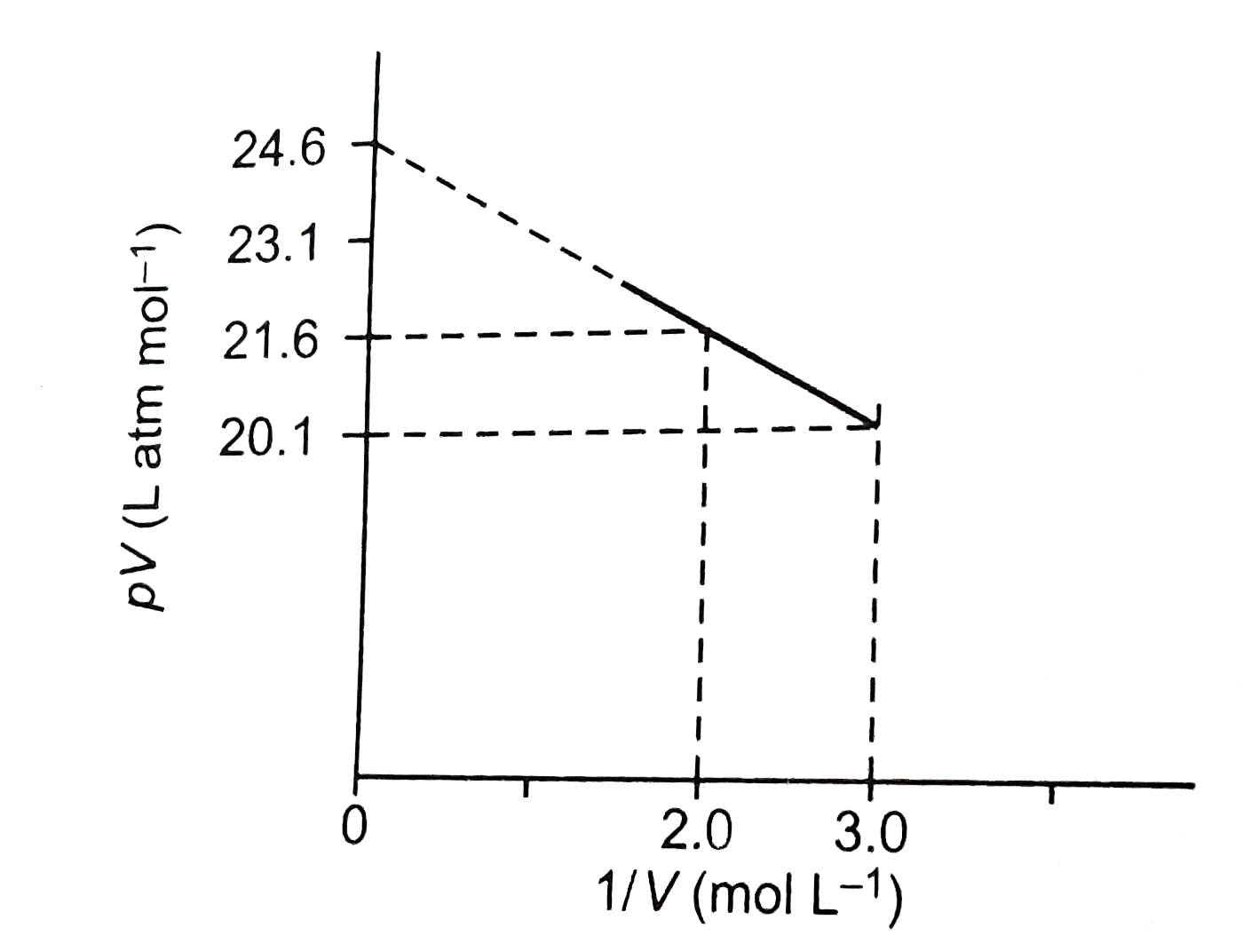
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12
The value of compression factor at the critical state of a vander waals gas is

Van Der Waals Equation of State - an overview
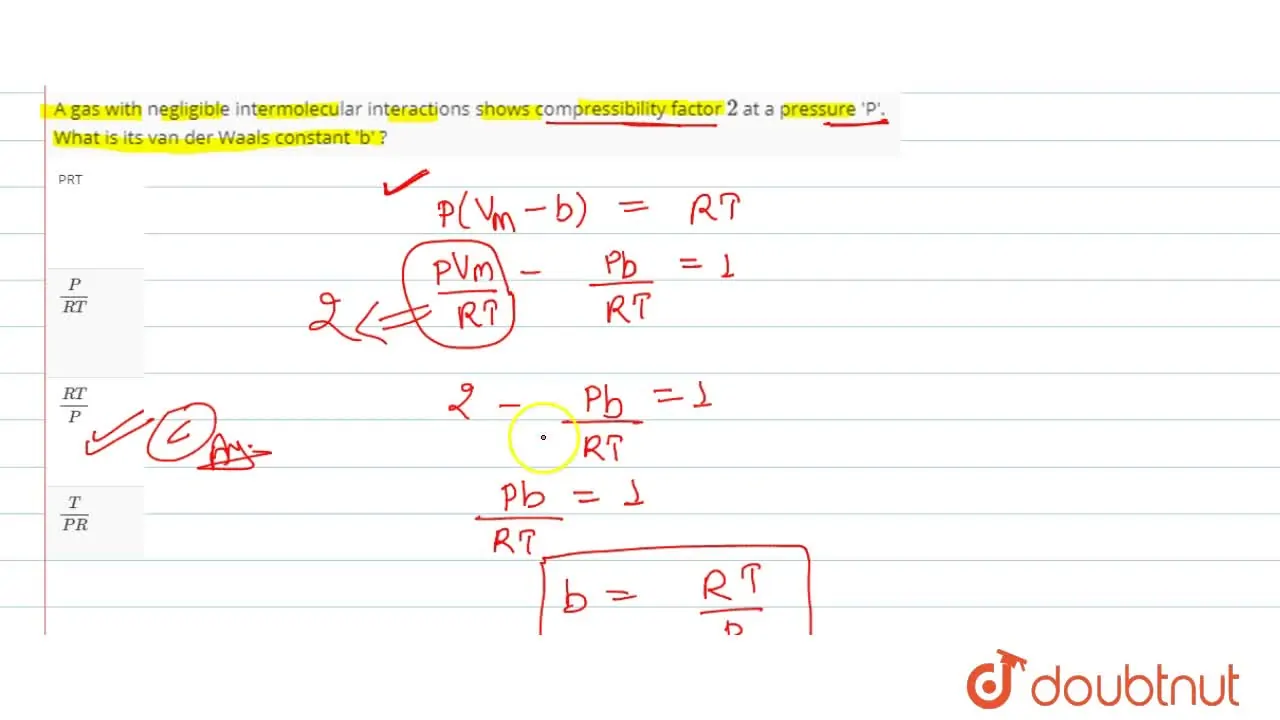
A gas with negligible intermolecular interactions shows compressibilit
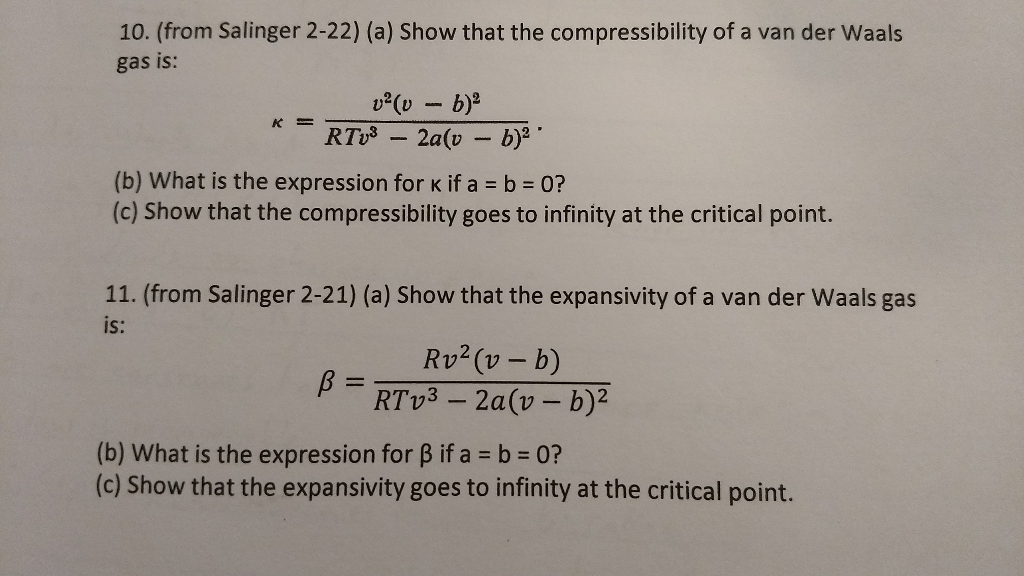
Solved (a) Show that the compressibility of a van der Waals
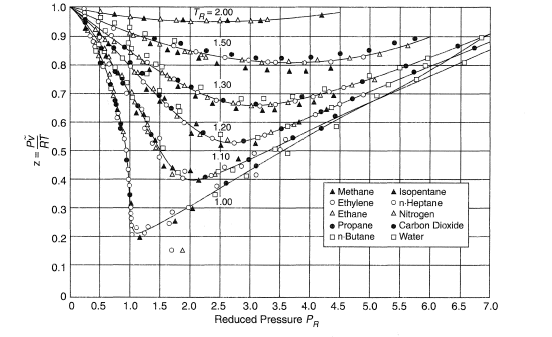
GAS LAW
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions

The compressibility factor of a van der Waals gas the critical point is equal to

Solved Data: Ideal gas constant R 8.314 J mol-1 K-1 1(a)