The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..

Solutions (1-47) - Final, PDF, Solubility
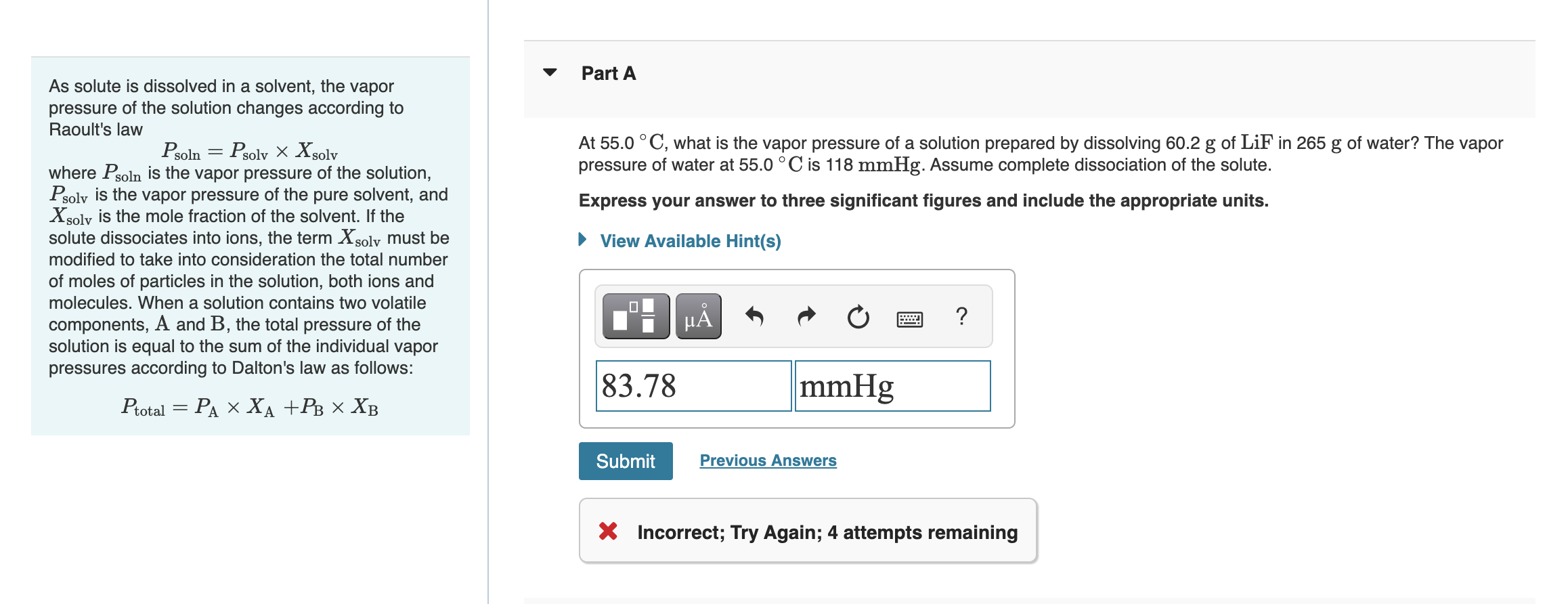
Solved As solute is dissolved in a solvent, the vapor
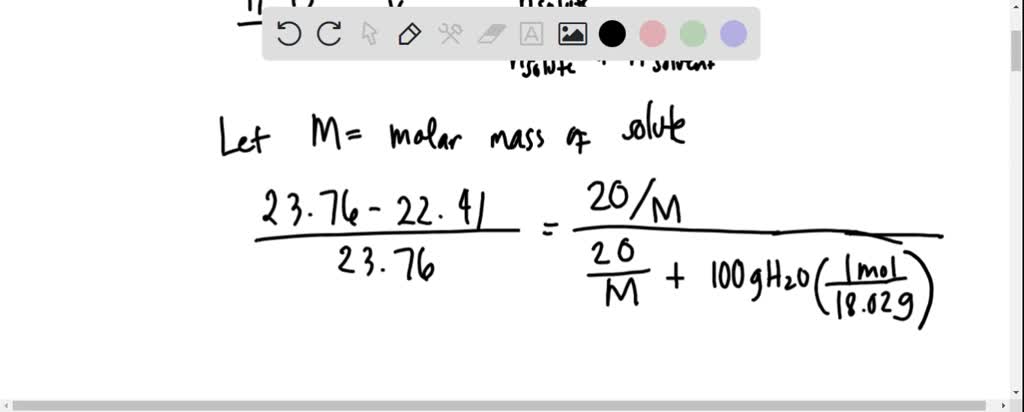
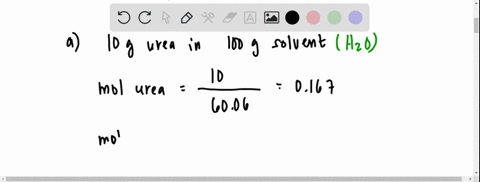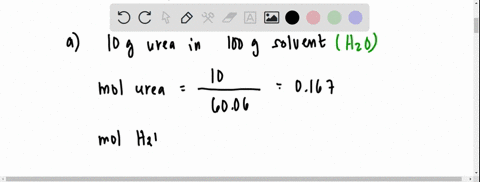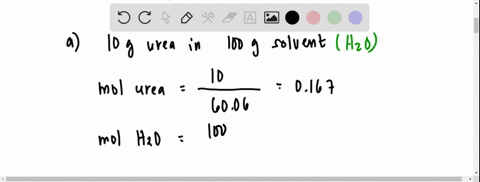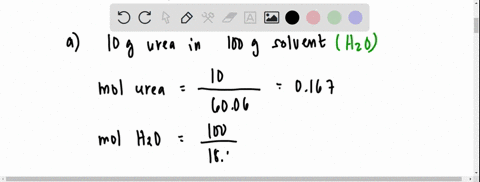
SOLVED: 20 g of solute was added to 100 g of water at 25^oC . The vapour pressure of water and that of solution were 23.76 mmHg and 22.41 mmHg respectively at

The vapour pressure of `CS_(2)` at `50^(@)C` is `854 torr` and a solution of `2.0 g` sulphur in

Fundamentals of General, Organic, and Biological Chemistry 9780134015187, 129212346X, 9781292123462, 0671599941, 0786821124, 0134015185
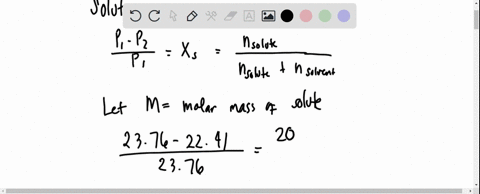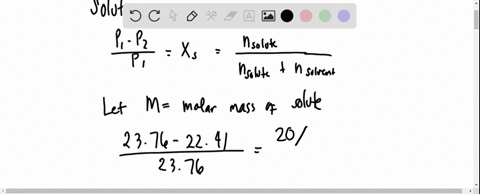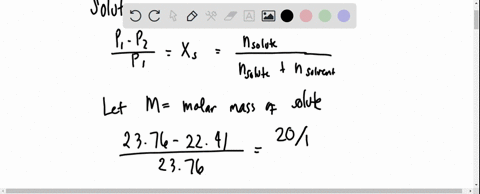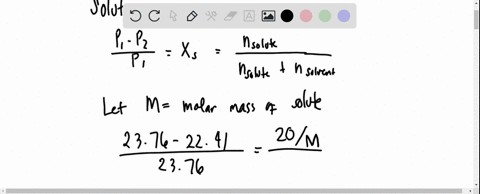
SOLVED: The vapour pressure of a solution having 2.0 g of solute X

Chapter-10 Solutions.pdf - Chemistry - Notes - Teachmint

fraction olligative Properties, Abnormality in Molar Mass) uids has a boi..
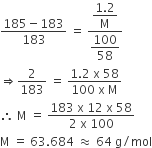
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o

9) The vapour pressure of CS2at 5000C is 854 mm Hg .A solution of 2.0g sulphur in 100g of CS2 has a vapour
SOLVED: The vapour pressure of a solution having 2.0 g of solute X
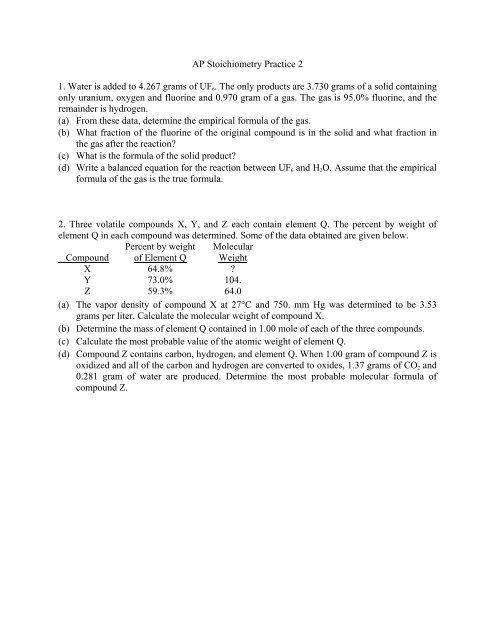
Stoichiometry Practice 2 answer key

my lioperties, Abnormality in Molar Mass) 2. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g vapour pressure =