Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an

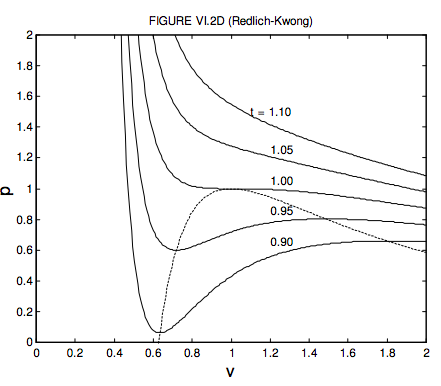
6.3: Van der Waals and Other Gases - Physics LibreTexts
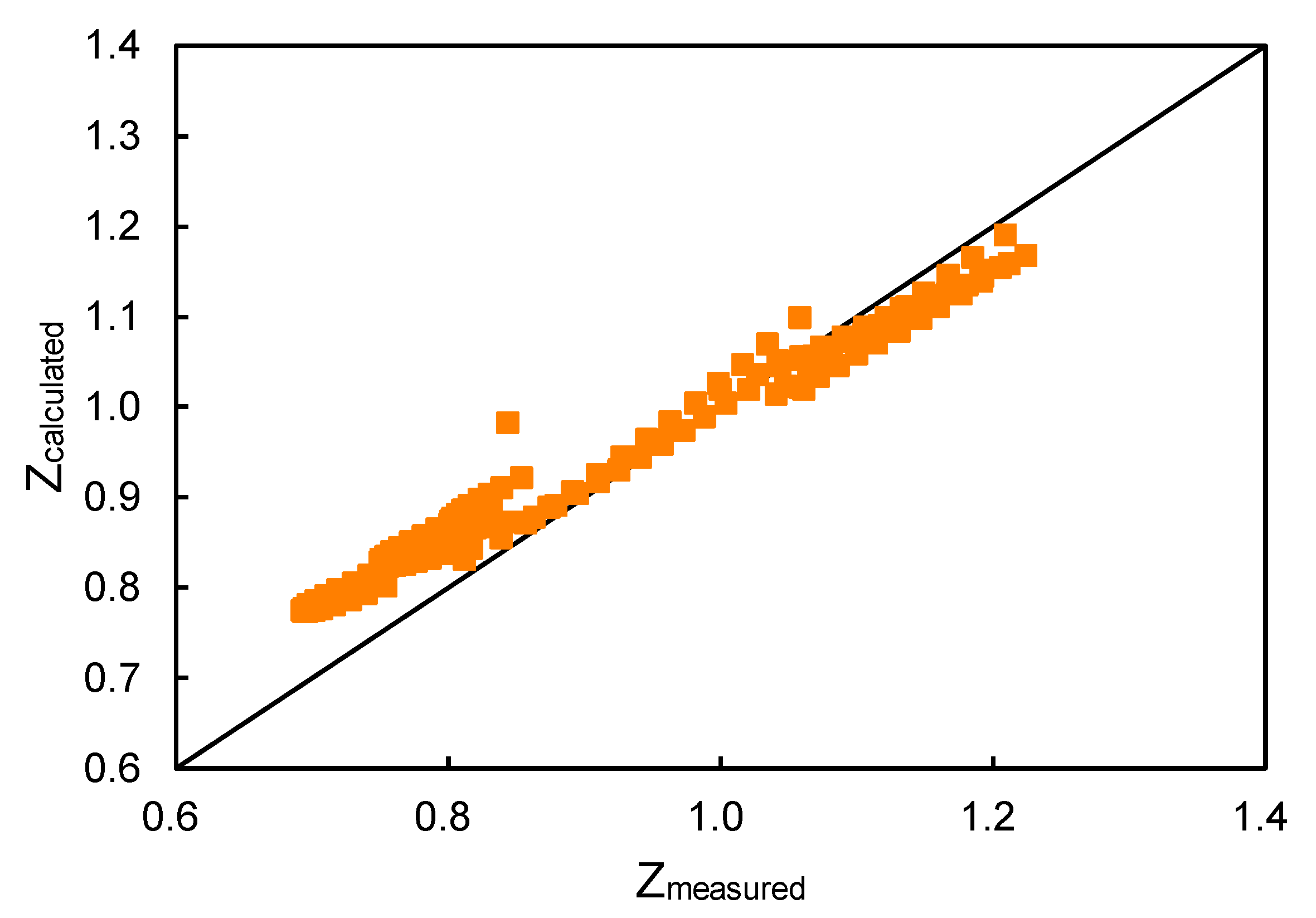
Energies, Free Full-Text

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
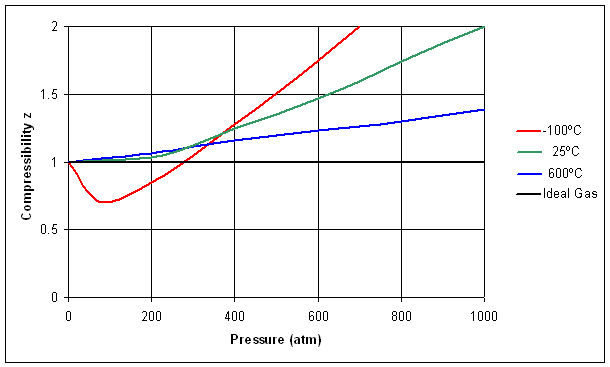
Gas Laws – First Year General Chemistry

Van der Waals equation, when pressure correction is ignored, one mole can be written as P(V - b) = RT. The correct expression compressibility factor will be
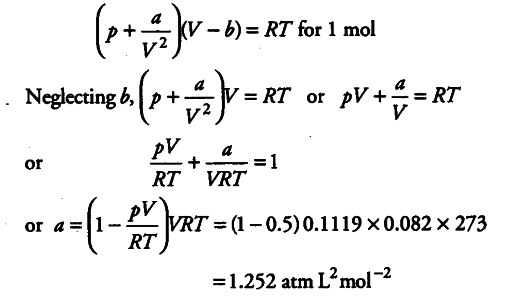
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum
If one mole of monoatomic gas is mixed with one mole of diatomic gas, what is the value of (Cp/Cv) for the mixture? - Quora

Solve this: Q) At the critical point for H2 gas, the value of compressibility factor, z=38, then the - Chemistry - States of Matter - 11917201

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application