Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

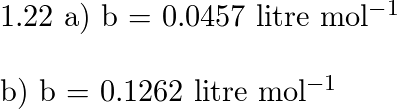
a) A certain gas obeys the van der Waals equation with $a =
List references from the University of Geneva Physical Chemistry reference database

the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5
Could 25 g of argon gas in a vessel of volume 1.5 dm3 exert a pressure of 2.0 bar at 30°C if it behaved as a perfect gas? If not, what pressure

1148 questions with answers in GAS
Why is the calculated pressure of a gas assuming ideal gas behavior different from one assuming the van der Waals equation? - Quora

I need help with question 3: a,b,c, i'm stuck and

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

At high pressure, the compressibility factor for one mole of van der w

The compression factor (compressibility factor) one mole of a van der Waals'gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible

1148 questions with answers in GAS


Graeff's experiments and 2LoD: Replication and Implications