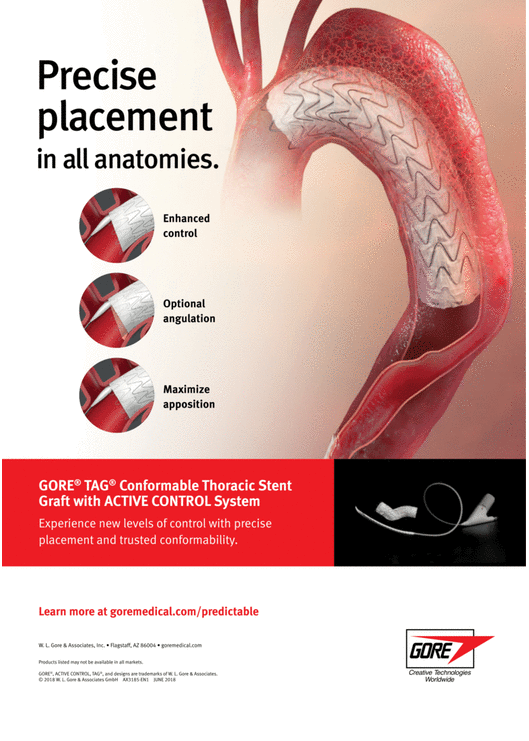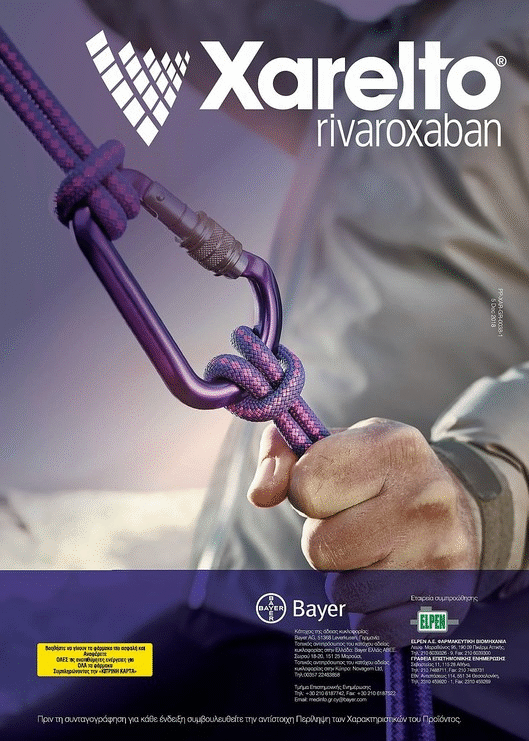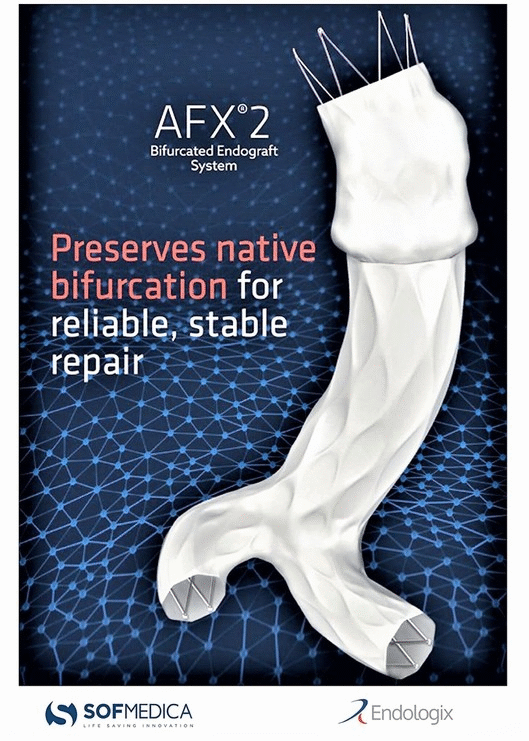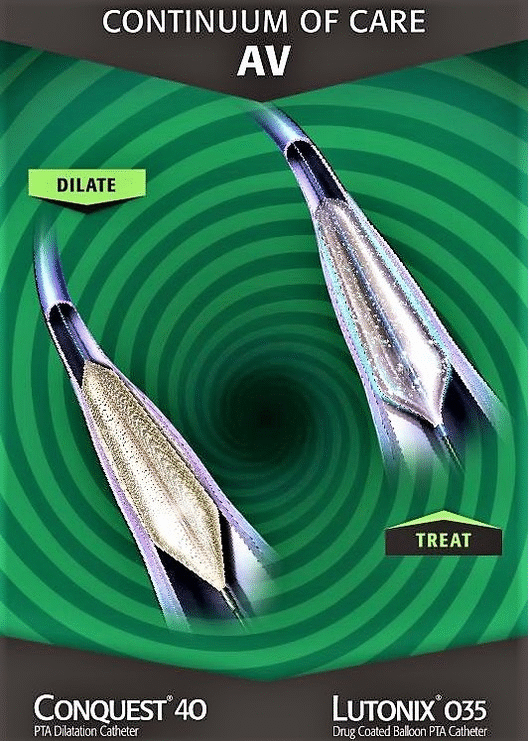
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL

Combining a durable, proven stent graft with a delivery system that offers controlled, staged deployment for the endovascular repair of aneurysms, transections and Type B dissections of the thoracic aorta.
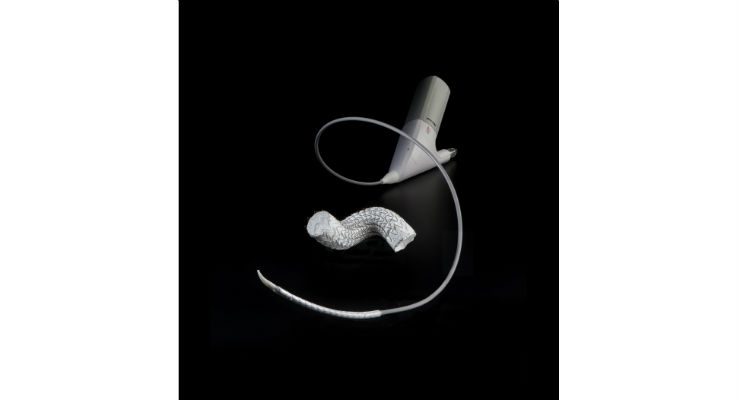
FDA Approves The GORE TAG Conformable Thoracic Stent Graft With ACTIVE CONTROL

GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System - Gore - PDF Catalogs

Anna Wieshaider% NEW_23.April.2021_ENACTIVECONTROLEVARTEVARTeaserShortFNL, Watch the on demand webinars now! Register now: #CX2021 #GoreMedical #vascular Sponsored by Gore
journal info – Heljves Hellenic Journal of Vascular and
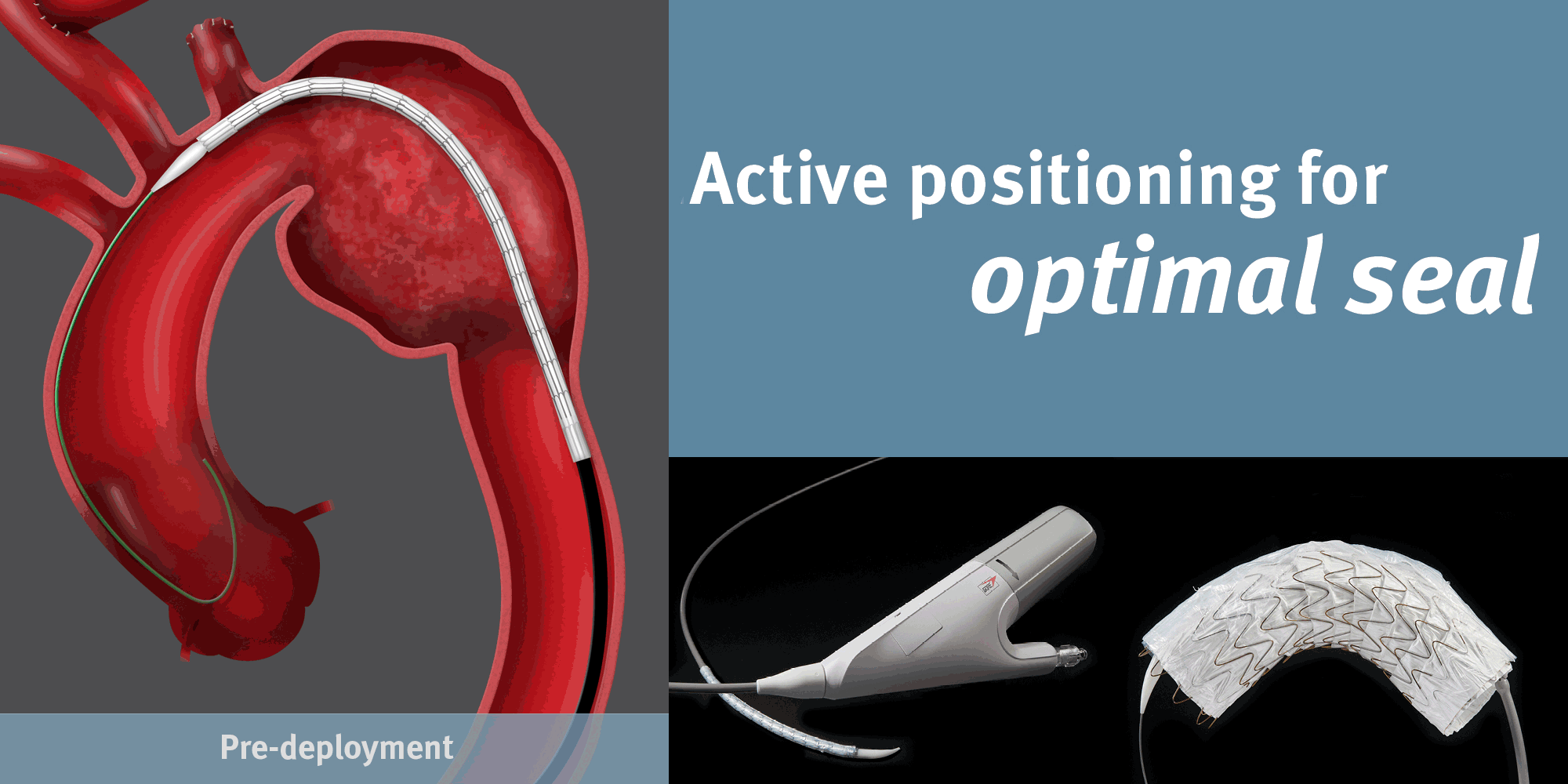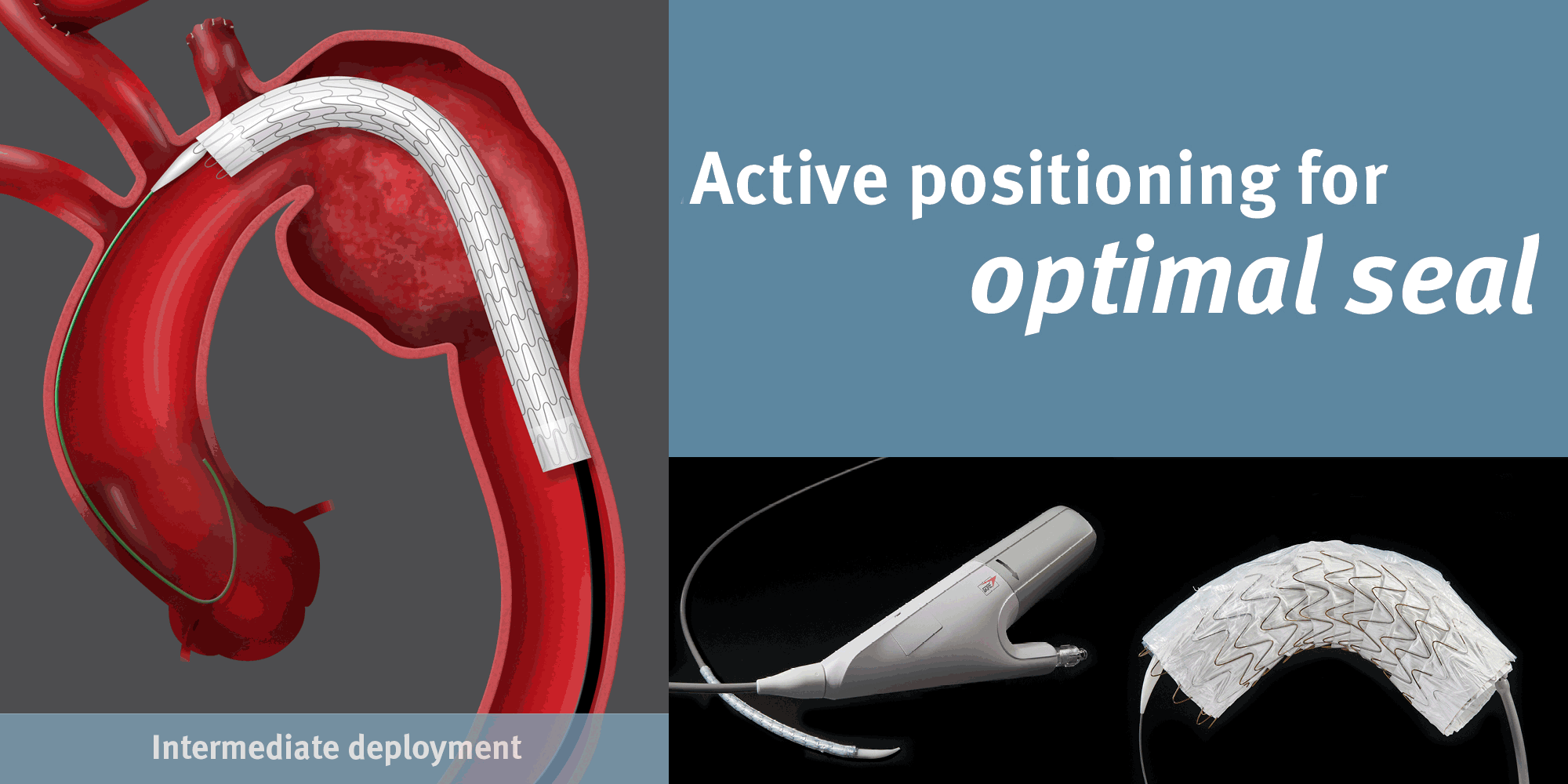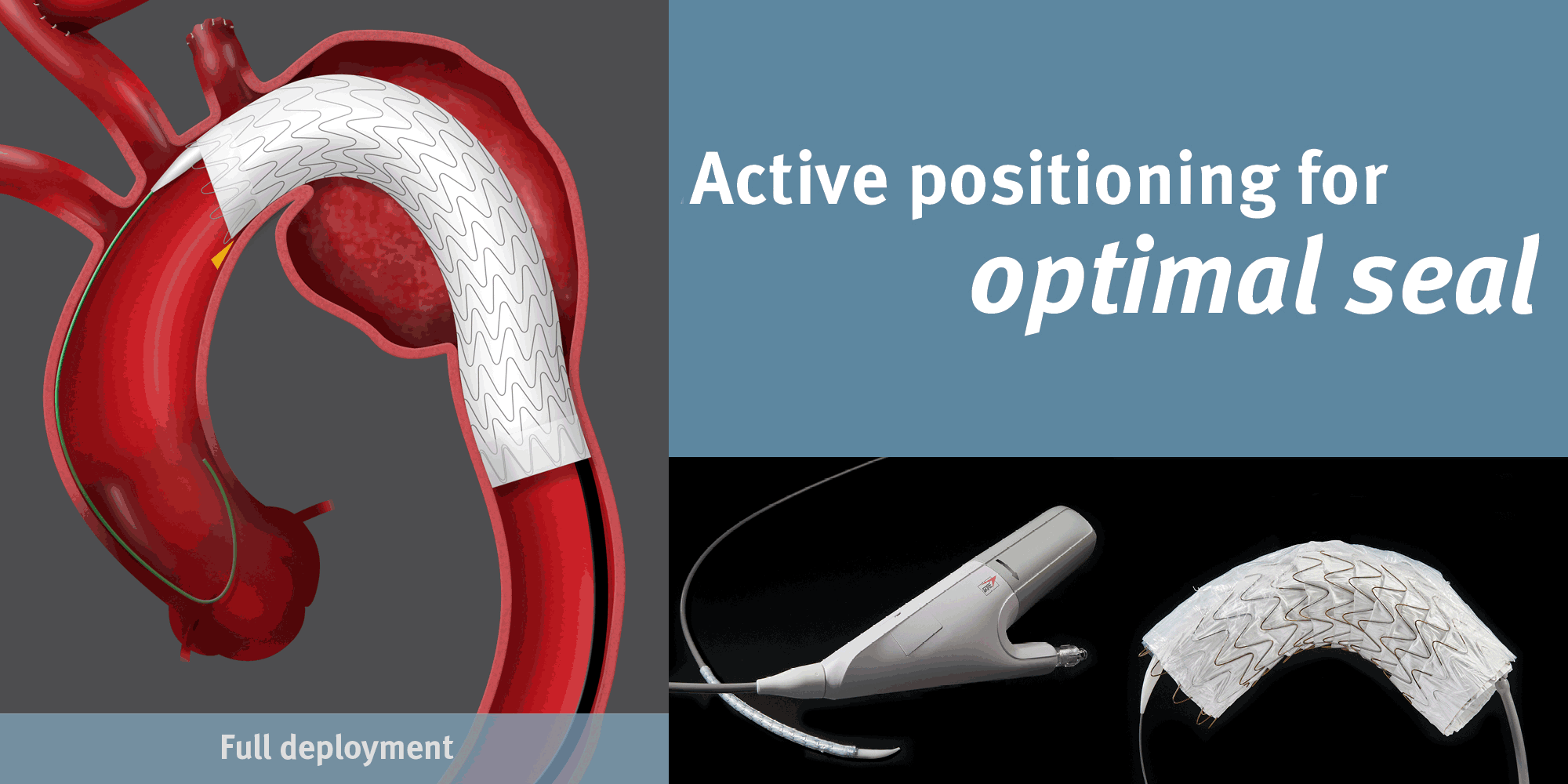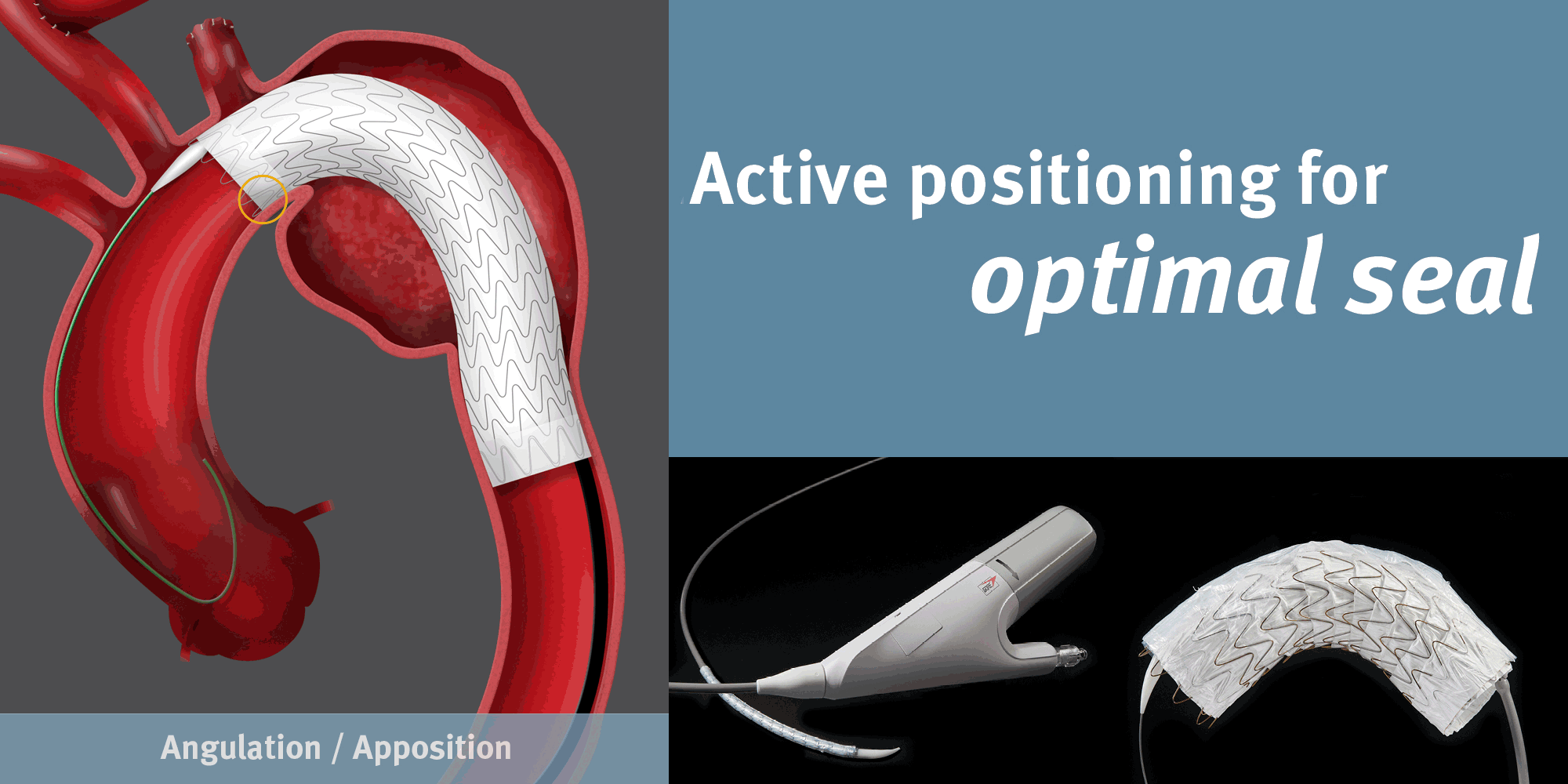
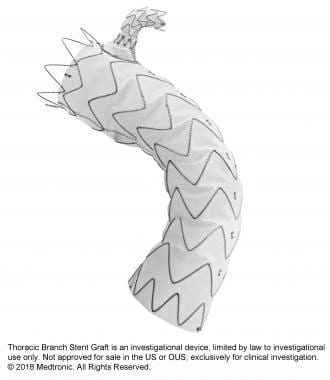
GORE® TAG® Conformable Thoracic Stent Graft

Conformable GORE® TAG® Thoracic Endoprosthesis with ACTIVE CONTROL System

Early Experience Using the GORE® TAG® Conformable Thoracic Stent

GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System – Vascupedia
Thoracic Endovascular Aortic Repair (TEVAR): Background, Indications, Contraindications
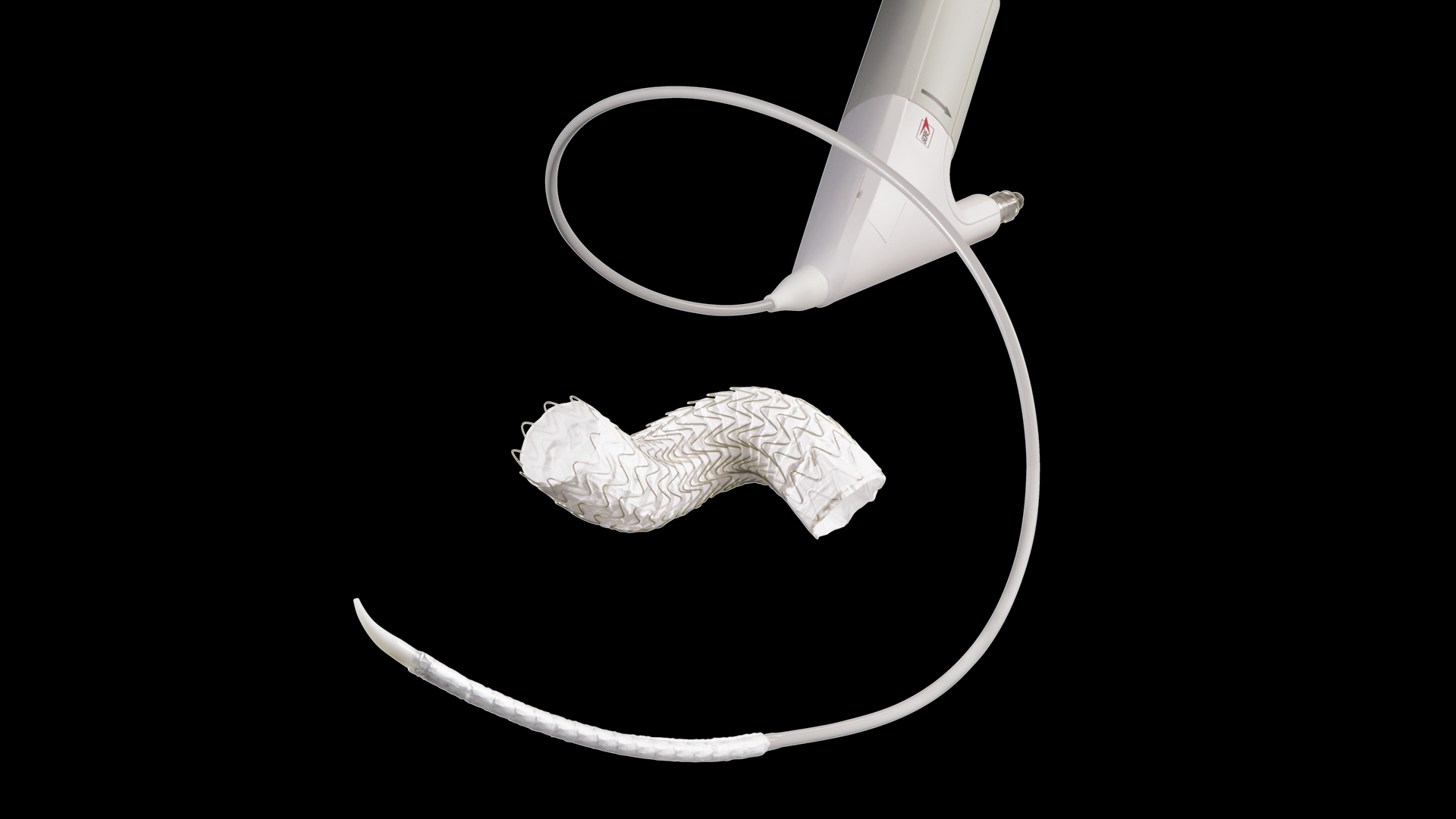
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System – Vascupedia

GORE cTAG-The evolution of Gore's TEVAR device, from the original GORE

Role of Endoluminal Techniques in the Management of Chronic Type B Aortic Dissection
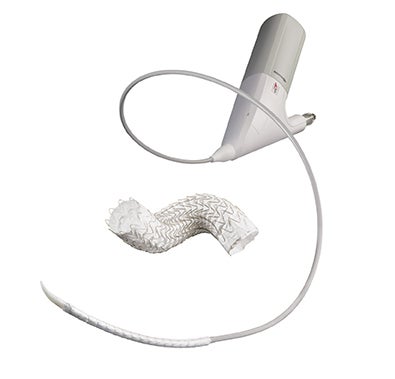
Product Value—GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
Achieving Optimal TEVAR Positioning: Deployment Example in Type B Dissection