The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a

What is the compressibility factor Z for 0.02 mole of a van der waal's gas at pressure of 0.1 atm. Assume the size of gas molecule is negligible. Given: RT =20 L
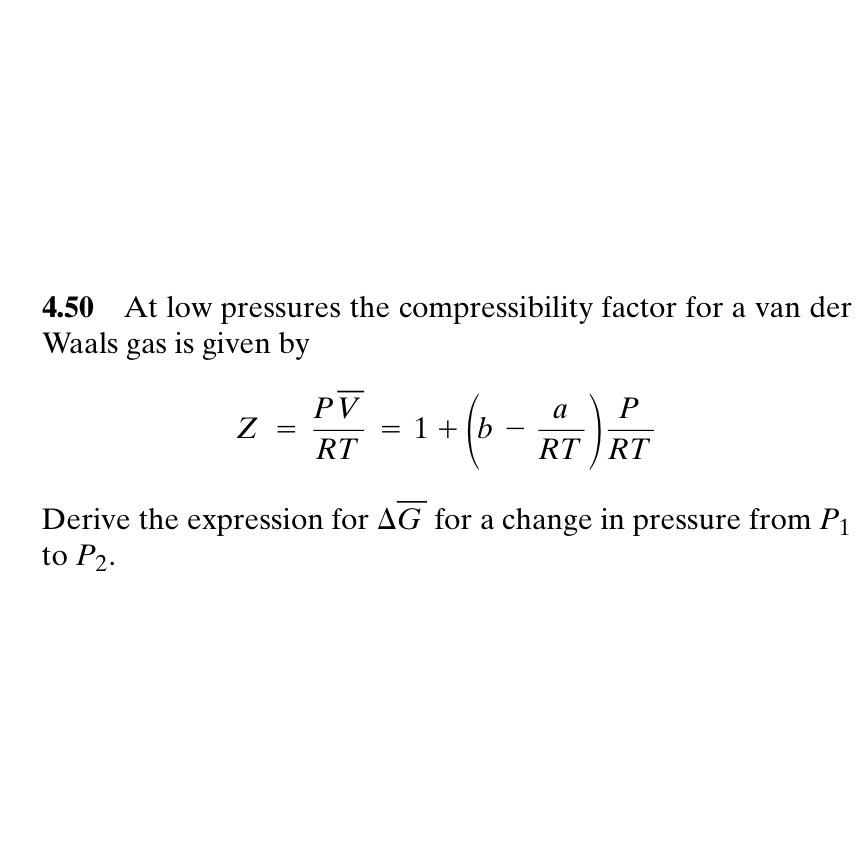
Solved 4.50 At low pressures the compressibility factor for
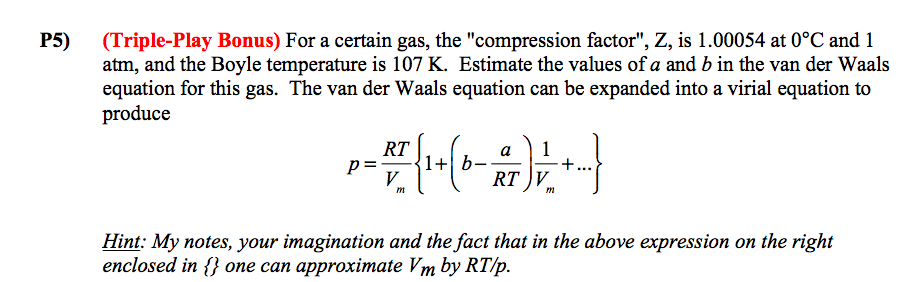
Solved (Triple-Play Bonus) For a certain gas, the


The compressibility factor 1 mole of Vander Waal's gas 0^{o}C and 100 atm pressure is 0.447. Assuming the volume of gas molecules negligible, the value of Vander Waal's constant 'a' is: 1.24

If `Z` is a compressibility factor, van der Waals' equation at low

Djective lype Questions The compressibility factor of N2 moderate pressure range is equal to [where a & b are the van der Waal's constants] Pb Pb (2) 1- (1)RT RTV (3) (1RTV
Bengali] The compresibility factor (Z) of one mole of a van der waals

Telugu] The compression factor for one mole of real gas at 0^@C and 1

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

Compressibility Chart - an overview

A gas has a compressibility factor of 0.5 and a molar volume of 0.4 dm3 mol− 1 at temperature of 800K

al Gases f.a What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given: RT =