Pharmaceuticals, Free Full-Text
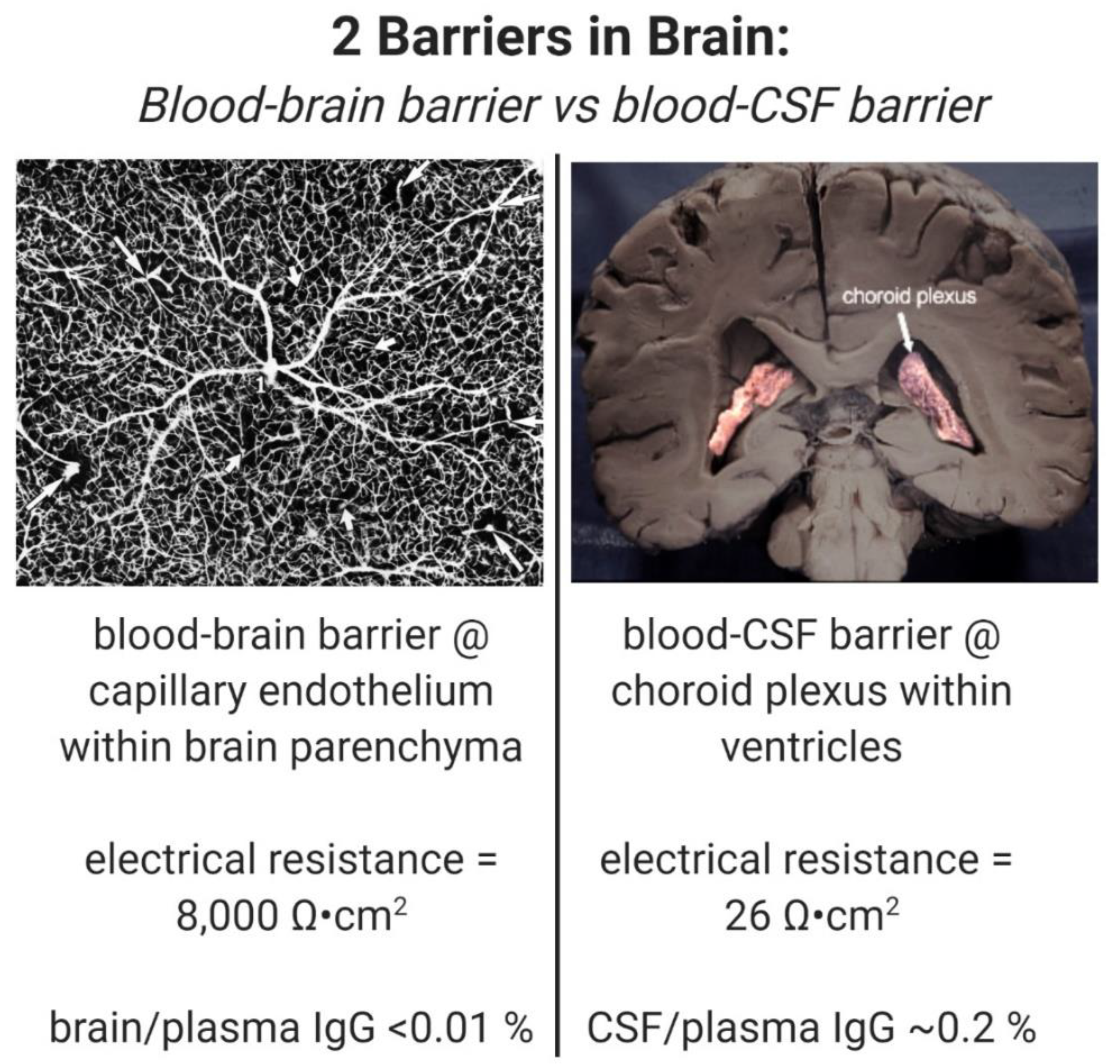
Despite the enormity of the societal and health burdens caused by Alzheimer’s disease (AD), there have been no FDA approvals for new therapeutics for AD since 2003. This profound lack of progress in treatment of AD is due to dual problems, both related to the blood–brain barrier (BBB). First, 98% of small molecule drugs do not cross the BBB, and ~100% of biologic drugs do not cross the BBB, so BBB drug delivery technology is needed in AD drug development. Second, the pharmaceutical industry has not developed BBB drug delivery technology, which would enable industry to invent new therapeutics for AD that actually penetrate into brain parenchyma from blood. In 2020, less than 1% of all AD drug development projects use a BBB drug delivery technology. The pathogenesis of AD involves chronic neuro-inflammation, the progressive deposition of insoluble amyloid-beta or tau aggregates, and neural degeneration. New drugs that both attack these multiple sites in AD, and that have been coupled with BBB drug delivery technology, can lead to new and effective treatments of this serious disorder.

Nitric Oxide Foundation - Berkeley Life Professional
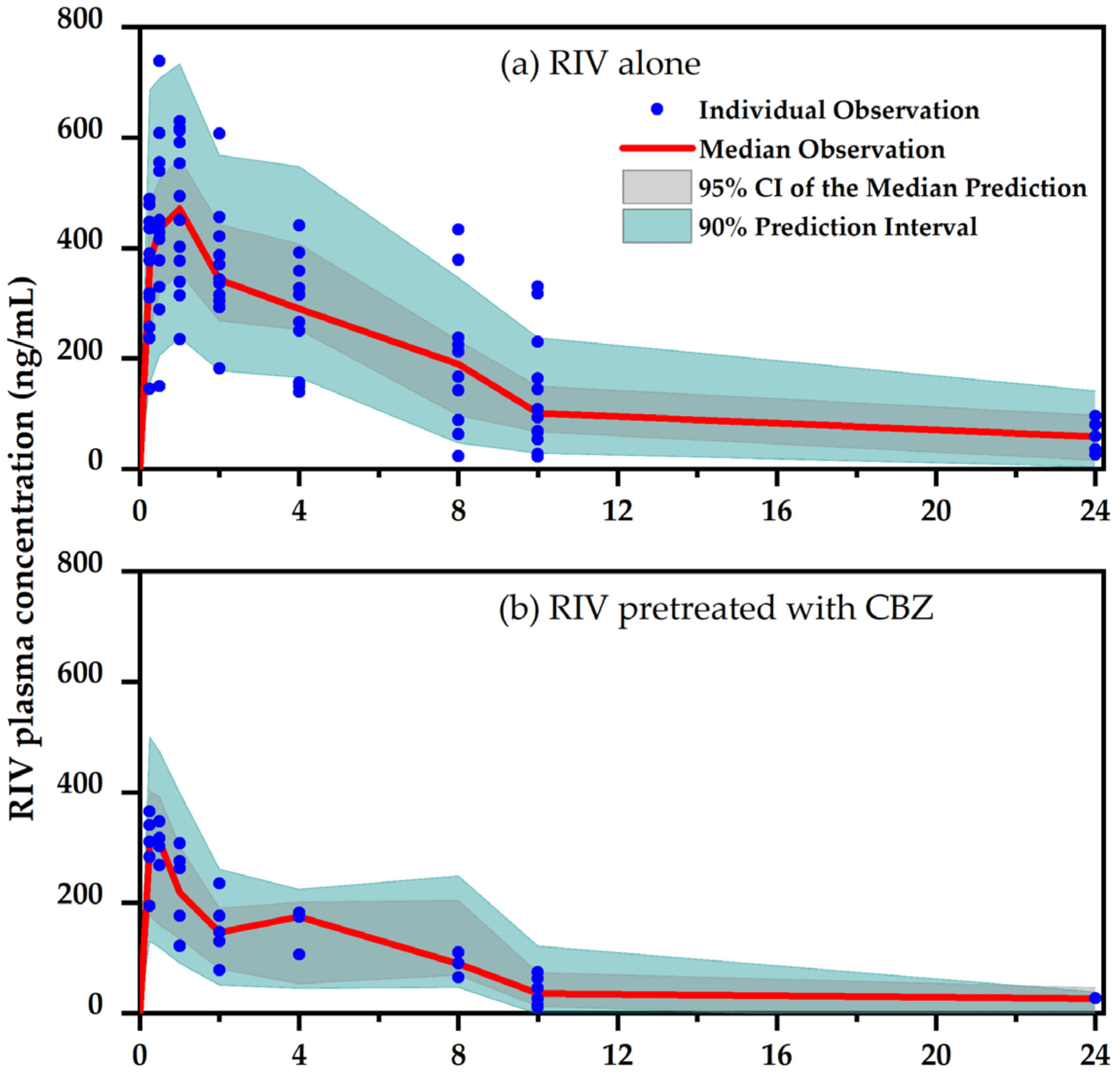
Pharmaceuticals, Free Full-Text
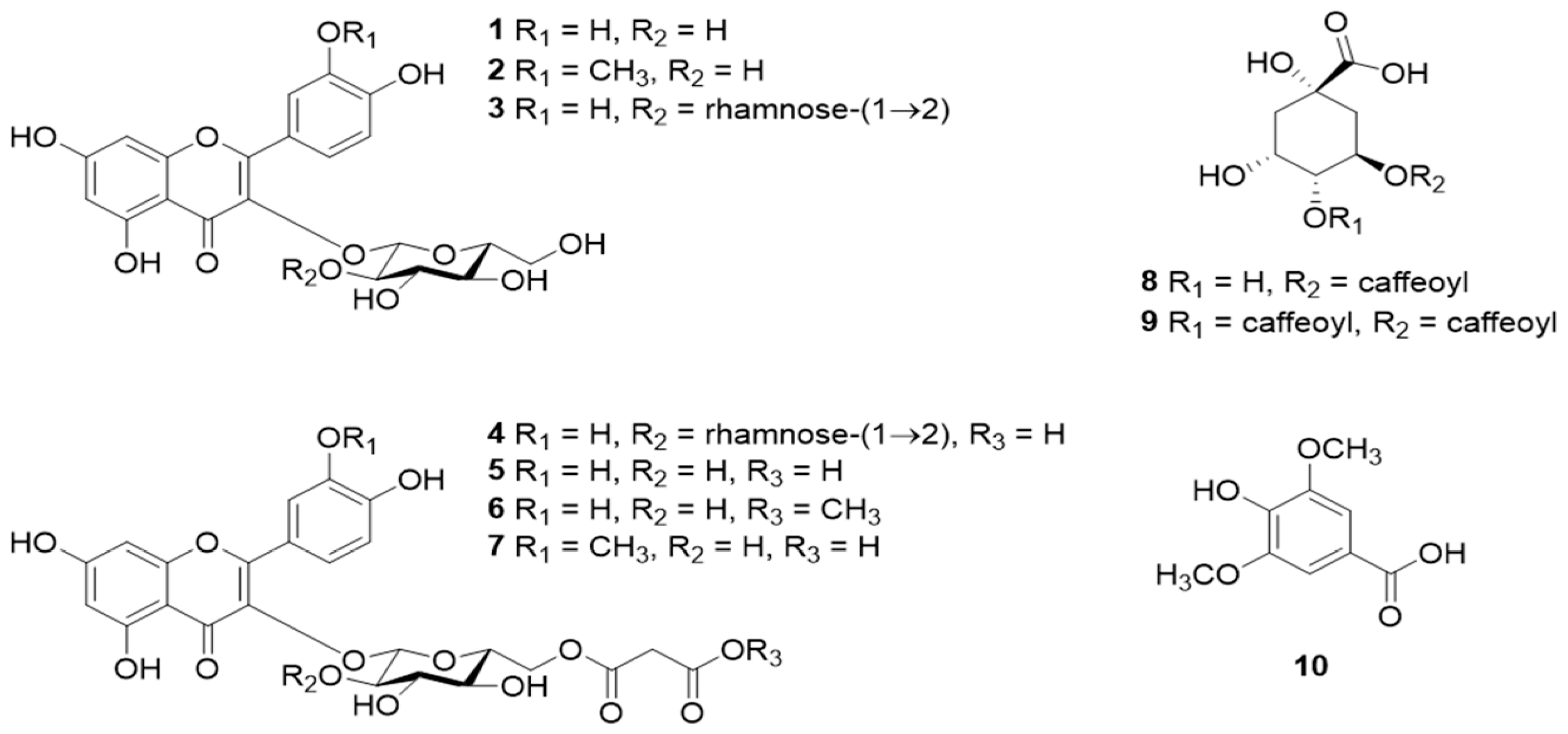
Pharmaceuticals, Free Full-Text

Pharmaceuticals, Free Full-Text

About Needle-free Injection Systems - PharmaJet

Home Pharmaceutical Research
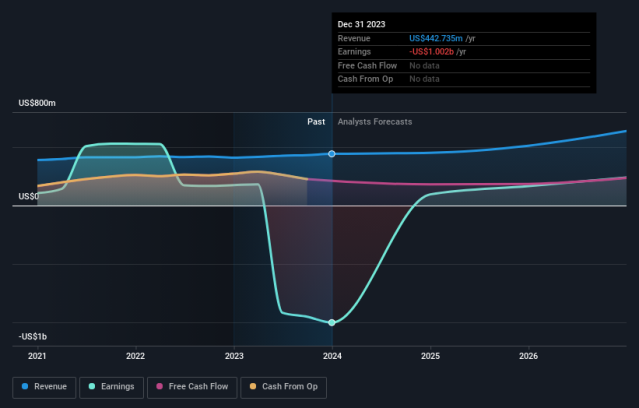
Ironwood Pharmaceuticals Full Year 2023 Earnings: EPS Misses Expectations

China SXT Pharmaceuticals Full Year 2023 Earnings: US$0.89 loss per share (vs US$5.52 loss in FY 2022)
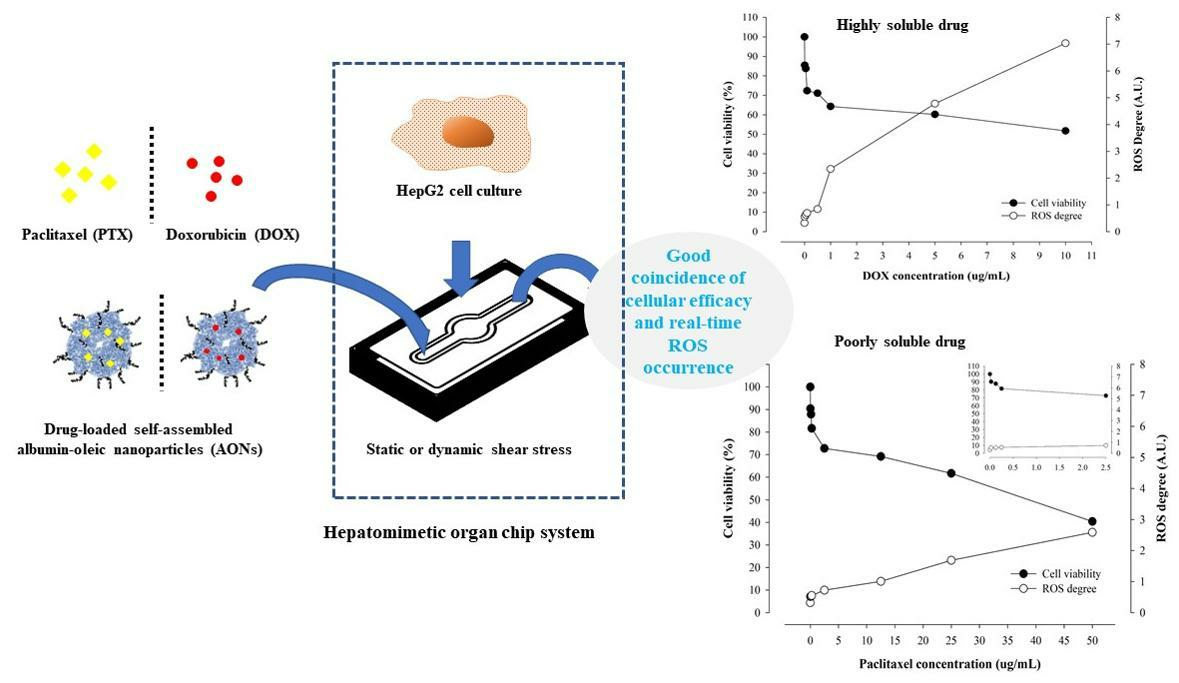
Pharmaceuticals, Free Full-Text

Pharmaceutical Press Essential Pharmaceutical Knowledge
:max_bytes(150000):strip_icc()/medicine-pills-463594335-ba46b2f34a764be6a9c0e56a308cb938.jpg)
Drug Classes and Medication Classification

Current Pharmaceutical Design
15,117 Global Pharmaceuticals Stock Photos, High-Res Pictures, and Images - Getty Images