Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel

Groundbreaking technology introduces increased control to achieve optimal coverage proven to significantly reduce the risk of toxicity to the rectum SANTA BARBARA, CALIF. / STOCKHOLM, SWEDEN – June 9, 2022— Palette Life Sciences, a fully-integrated global life sciences company dedicated to improving patient outcomes, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of […]
SpaceOAR - Augmenix, Boston Scientific, and Conflicts of Interest, Page 4

Dr. Martin King, MD – Boston, MA

Astrid Langoe on LinkedIn: I am happy to share that I have been selected as a student ambassador for…

Per-Olov Wedin posted on LinkedIn

益生菌活化出新技术,肠道存活率提高1000倍?
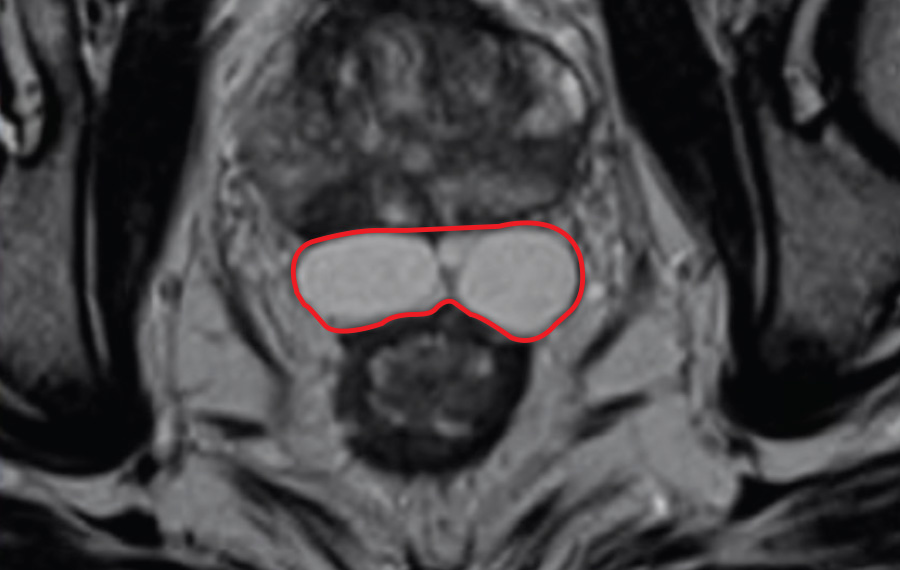
Control Matters Barrigel for Healthcare Providers

Dynamic Diagnostics Pre-filled 10% Neutral Buffered Formalin (NBF) Specimen
Control Matters Barrigel for Healthcare Providers
Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal Spacer, Proven Safe and Effective at Minimizing the Harmful Long-Term Side Effects of Prostate Radiation Therapy - Palette Life Sciences

Marianna Hawkins posted on LinkedIn

Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal Spacer, Proven Safe and Effective at Minimizing the Harmful Long-Term Side Effects of Prostate Radiation Therapy

David Aguilar on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…

Dr. Martin King, MD – Boston, MA

Vinny Devany on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…

Per Langoe on LinkedIn: #kudos #goingaboveandbeyond