Non-ideal behavior of gases (article)


SOLVED: The van der Waals equation compensates for non-ideal behavior of gases by taking into account intermolecular forces and the volume of the gas molecules. Not all gases behave ideally: An ideal

5 Ways to Get Students Energized About Ideal Gas Law
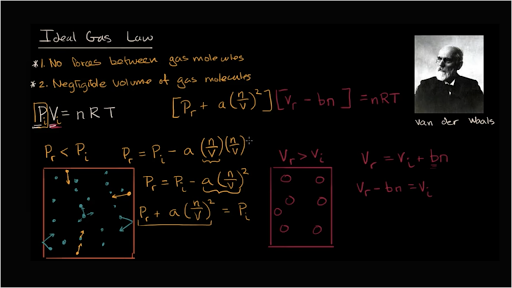
The van der Waals equation (video)

gas laws - Among hydrogen, helium and carbon dioxide, which gas would behave most like ideal gas and why? - Chemistry Stack Exchange
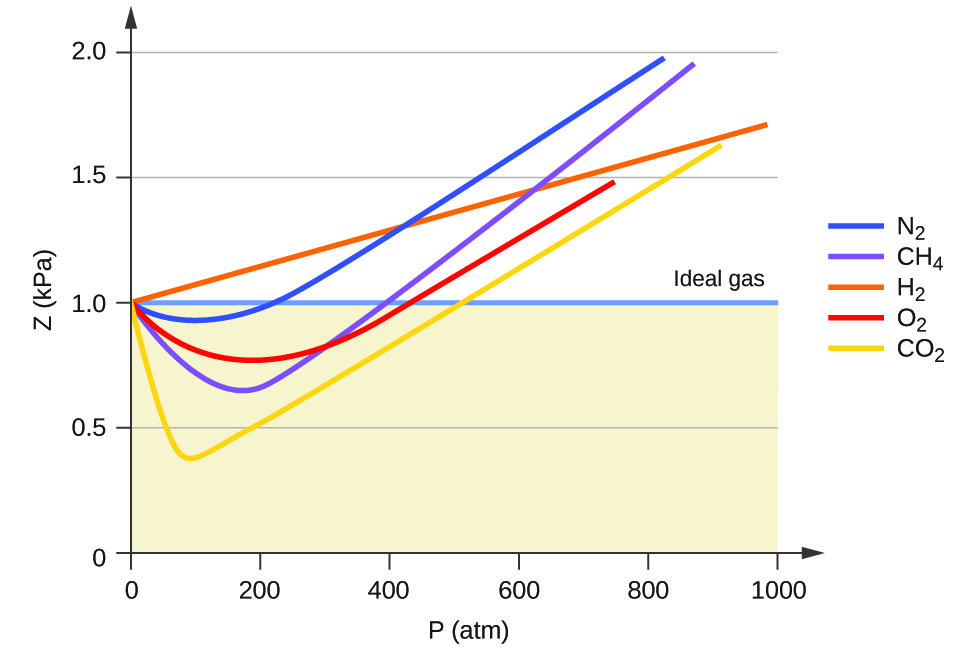
5.6 Non-Ideal Gas Behavior – Chemistry

Ideal gas law - Wikipedia
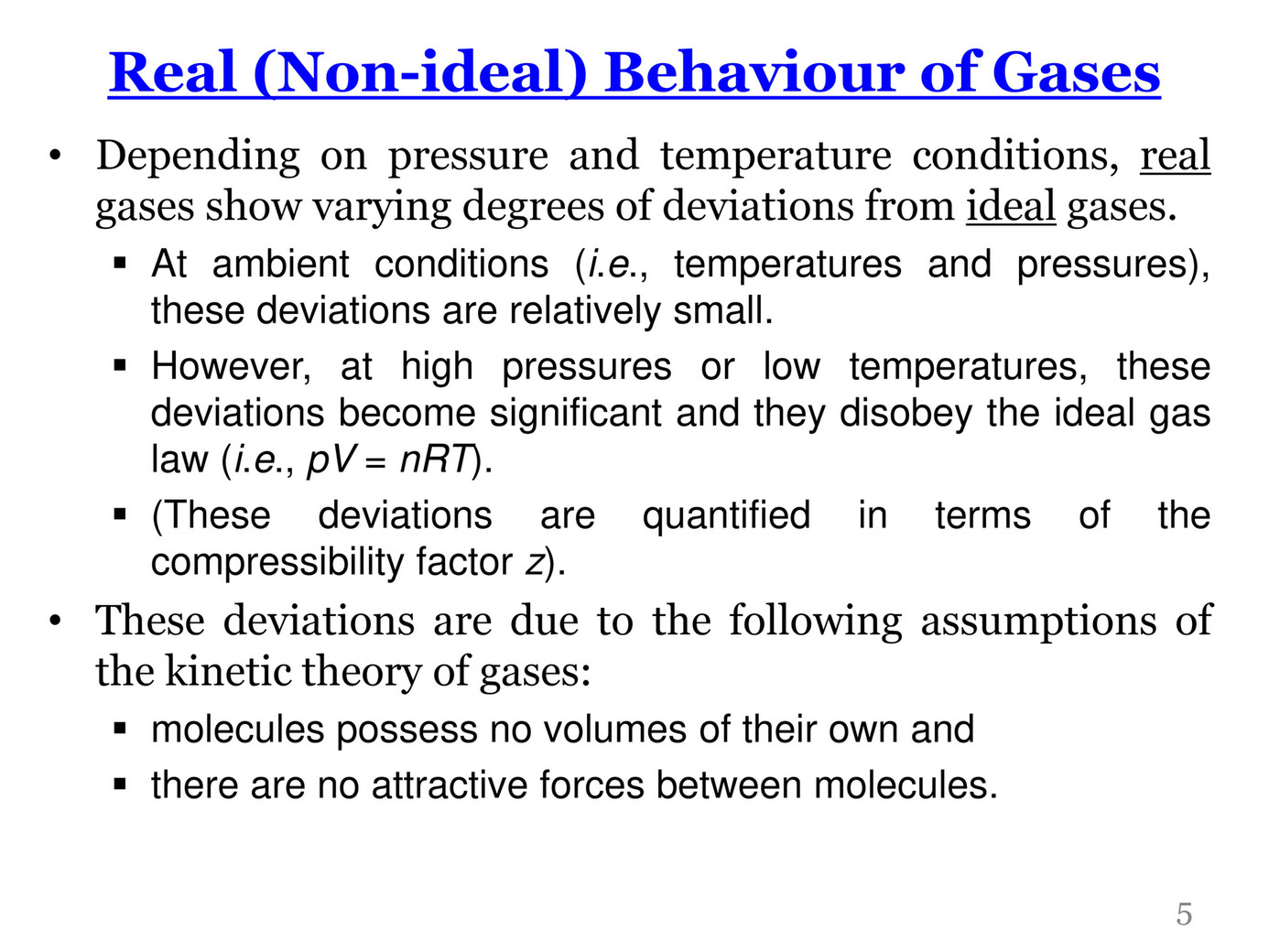
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com

Study the non-ideal behavior of gases - Property Packages Selection - Aspen Plus - Lecture # 35

Gases and kinetic molecular theory, Chemistry library