At 300 K, 36 g of glucose present per litre in its solution has an osm

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

An aqueous of glucose C(6)H(12)O(6) has an osmotic pressure of 2.72 at

Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. - Abstract - Europe PMC

Calculations Pharma, PDF, Solution

At `300 K`, `36 g` of glucose present per litre in its solution has an osmotic pressure of `4.98

Lab exam 1.pdf - VTPP 423 PHYSIOLOGICAL MEASUREMENTS Date 1/28/21 GOHIL AISHWA Name Lab Station 9A Kendall Amir Lab Partners

ANSWERED] At 300 K 36 g of glucose present in a litre of its solution - Kunduz

Diabetes insipidus in infants and children - ScienceDirect
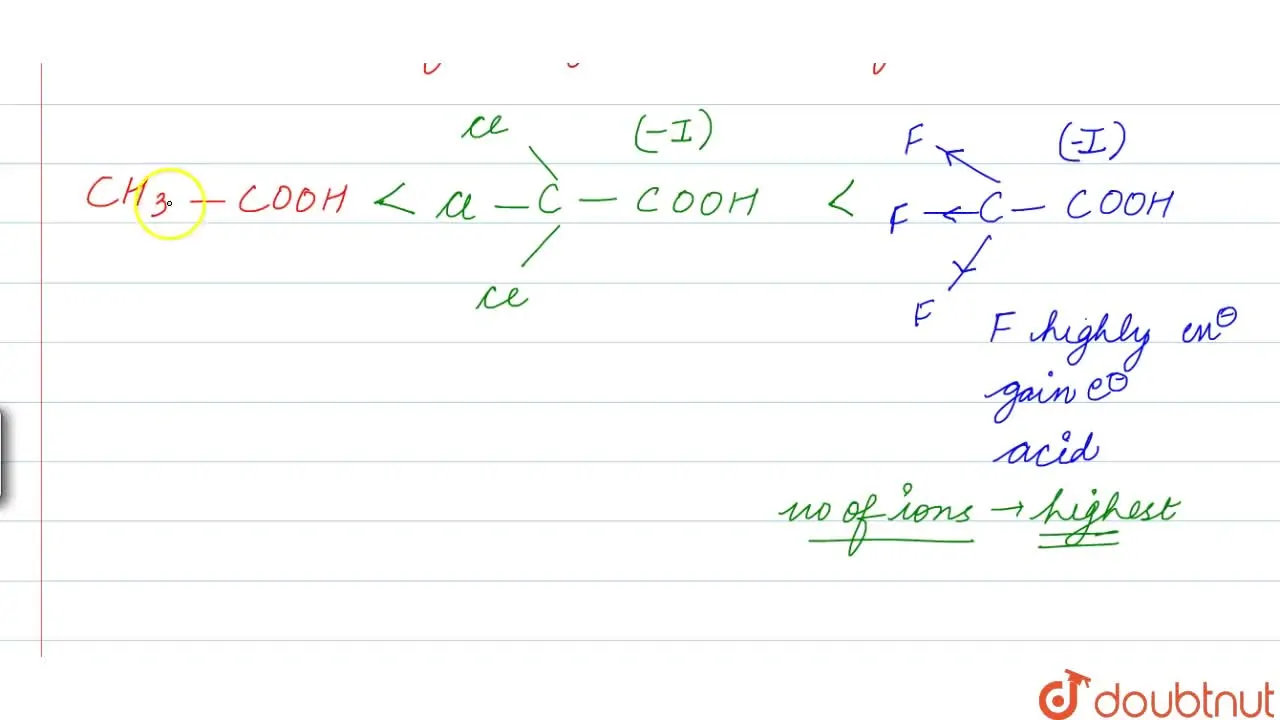
The depression in freezing point of water observed for the same amount

Why do gases always tend to be less soluble in liquids as the temperat

At `300 K`, `36 g` of glucose present per litre in its solution has an osmotic pressure of `4.98

State Henry's law and mention some important applications ?