Sacituzumab Earns Regular FDA Approval for TNBC - NCI

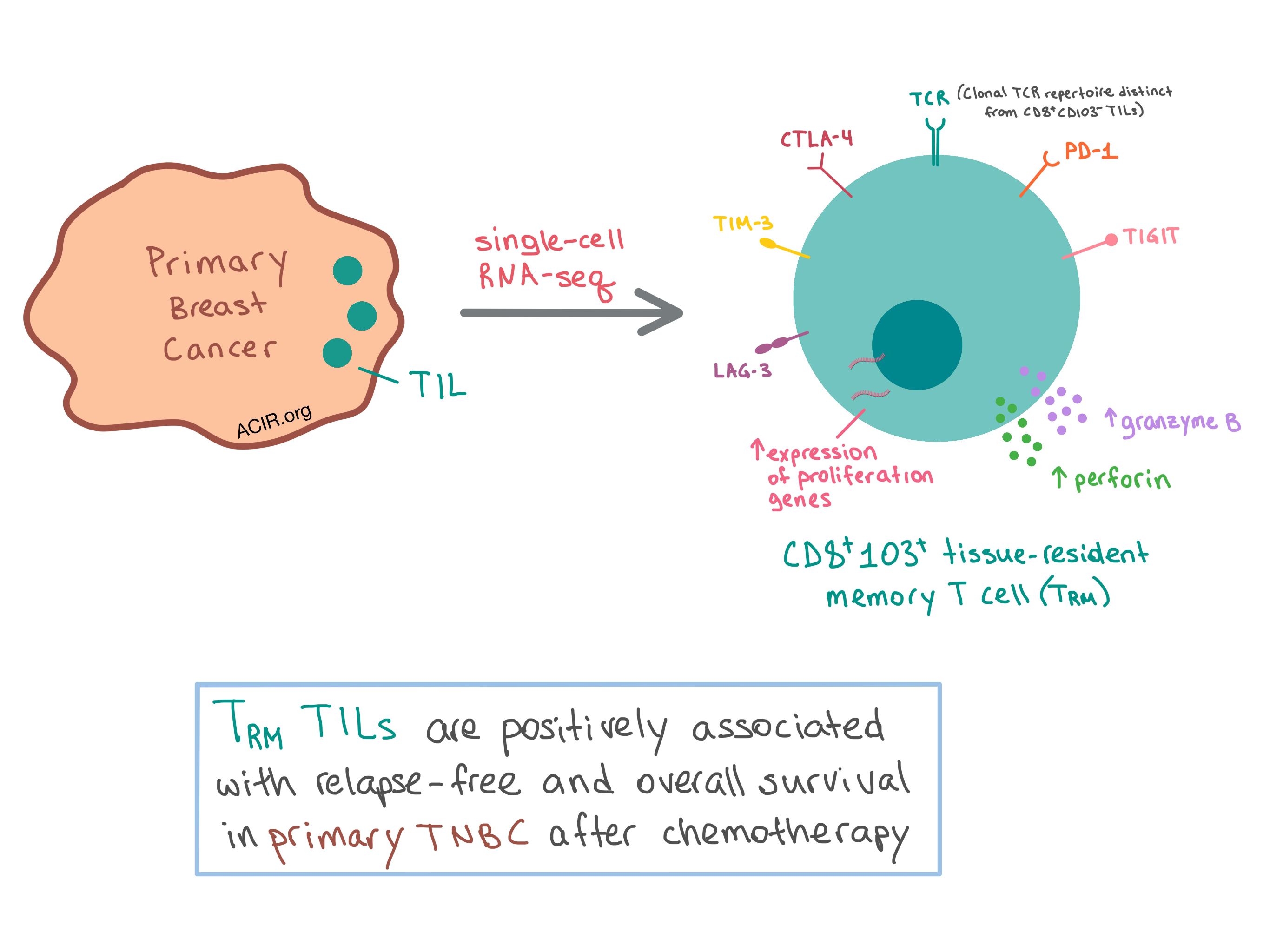
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.
Sacituzumab Earns Regular FDA Approval For TNBC NCI
View of Sacituzumab Govitecan (Trodelvy) Canadian Journal of Health Technologies
Trodelvy for the Treatment of Advanced Triple-Negative Breast Cancer

ADC Drug Trodelvy Shows Positive Efficacy In three Types of Cancers
FDA Approvals - Cancer Currents Blog - NCI

PDF) Challenges and Opportunities in Developing Targeted Therapies

Exploring the Potential of Metallodrugs as Chemotherapeutics for
Sacituzumab Earns Regular FDA Approval For TNBC NCI
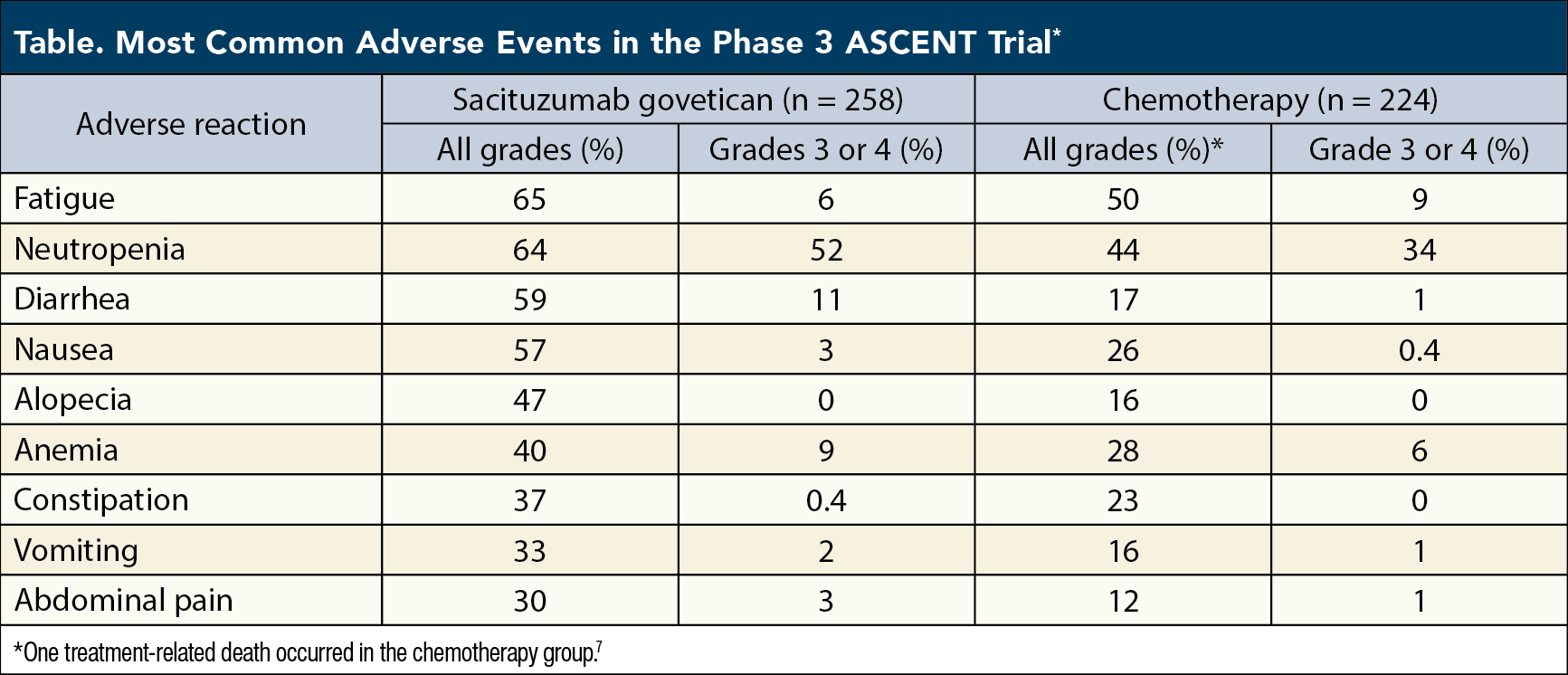
Sacituzumab Govitecan Moves to Second-Line Therapy for Metastatic

Kevin Punie on X: #ESMO21 Sacituzumab govitecan is a major
View of Sacituzumab Govitecan (Trodelvy) Canadian Journal of Health Technologies

Biomolecules, Free Full-Text

Sacituzumab Govitecan Moves to Second-Line Therapy for Metastatic
.jpg)
New FDA Alert Warns of Drug Combination for Advanced Triple-Negative Breast Cancer Patients