Solved 1. A cup of hot 50°C water is poured onto 35g of ice

Answer to Solved 1. A cup of hot 50°C water is poured onto 35g of ice
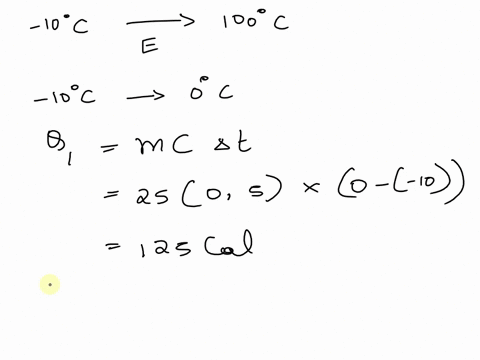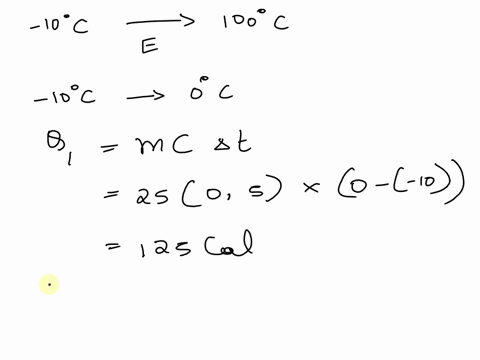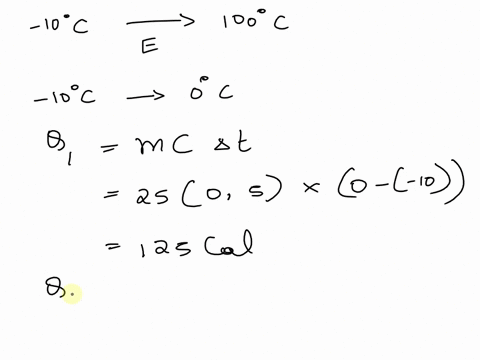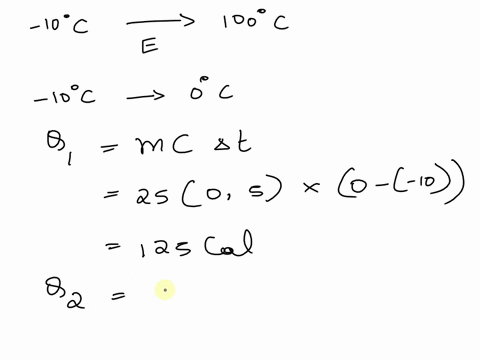
SOLVED: A cup of hot 50°C water is poured onto 35g of ice at 0°C until the entire system ends up at 20°C. How much energy is needed to melt the 35g

Specific Heat Capacity

A calorimeter of mass 50 g and specific heat capacity 0.42 J {g}^{-1} {℃ }^{ -1 } contains some mass of water {20}^{o}C. A metal piece of mass 20 g {100}^{o}C is

Heat and Temperature Change - ppt download
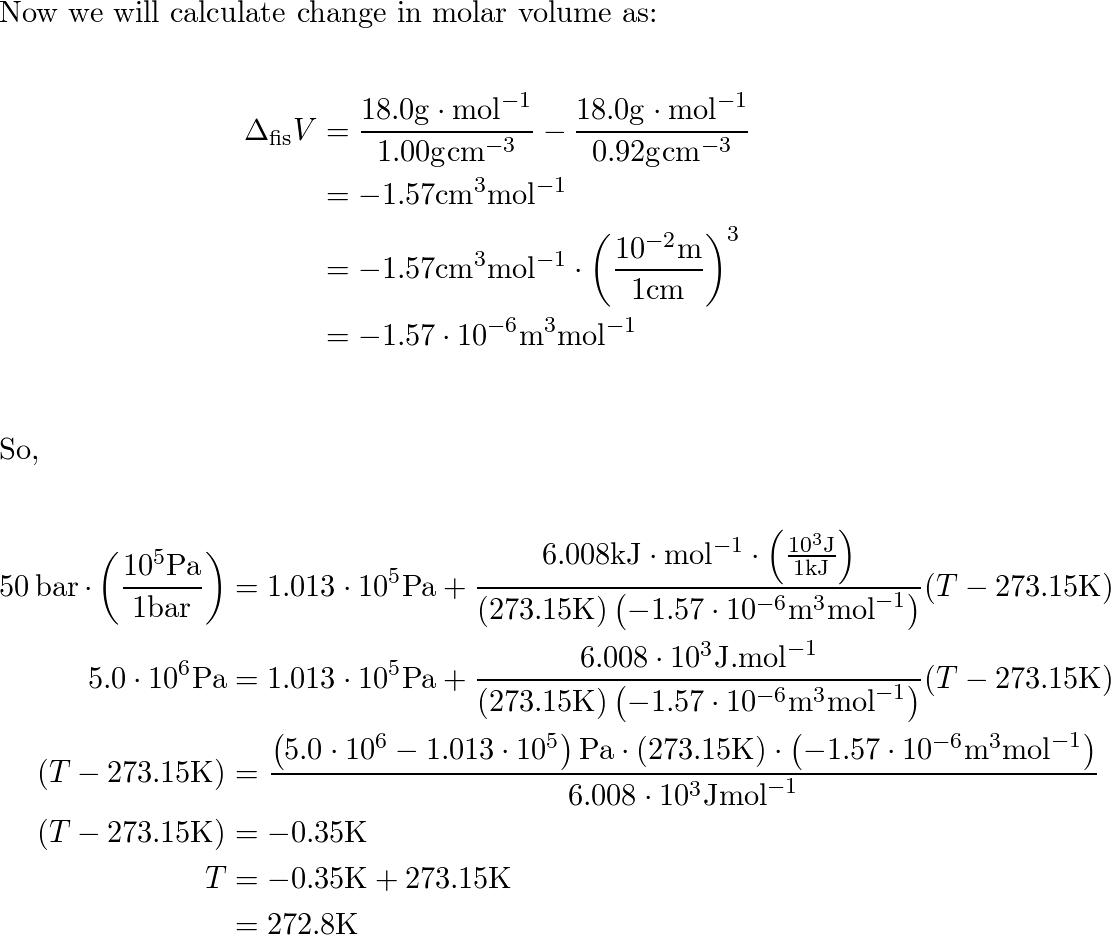
a) Estimate the melting point of ice under a pressure of 50

14.25 If you pour 0.0100 kg of 20.0ºC water onto a 1.20-kg block of ice (which is initially at

Biochemistry.pdf

First for Women January 16, 2023 (Digital)

Specific Heat Capacity

Solved EXERCISLU W PU EXAMPLE 14-7 ESTIMATE Will all the ice