Solved A 45-g block of copper at −12∘C is added to 120 g of
Answer to Solved A 45-g block of copper at −12∘C is added to 120 g of
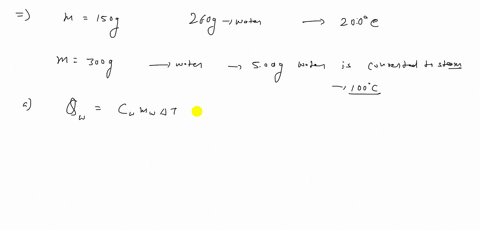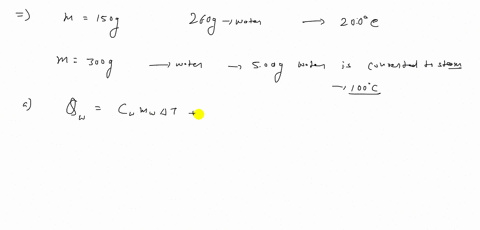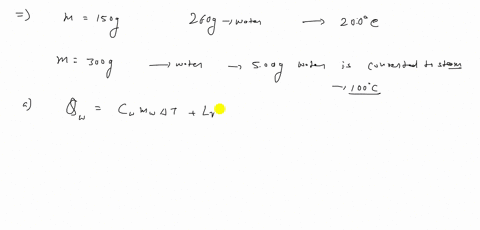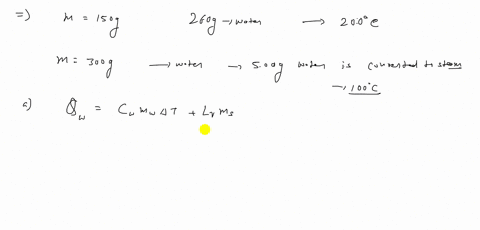
⏩SOLVED:A 150 g copper bowl contains 260 g of water, both at…
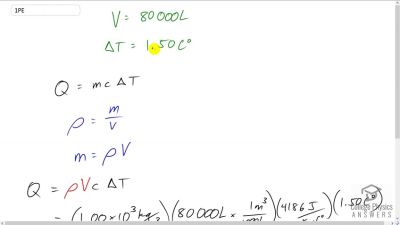
Chapter 14: Heat and Heat Transfer Methods
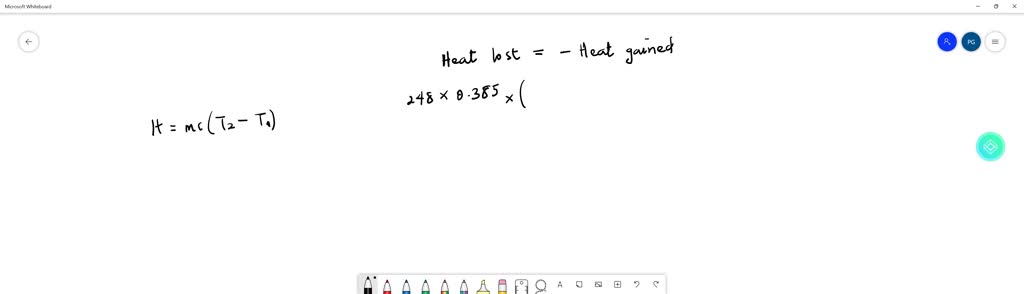
SOLVED: Use the following calorimetric values to answer the question: The specific heat capacity of water is 4,186 J/kg°C. The specific heat capacity for copper is 387 J/kg°C. A 120-g copper ball

Marathi] Solve the following problems : What will be the amount of
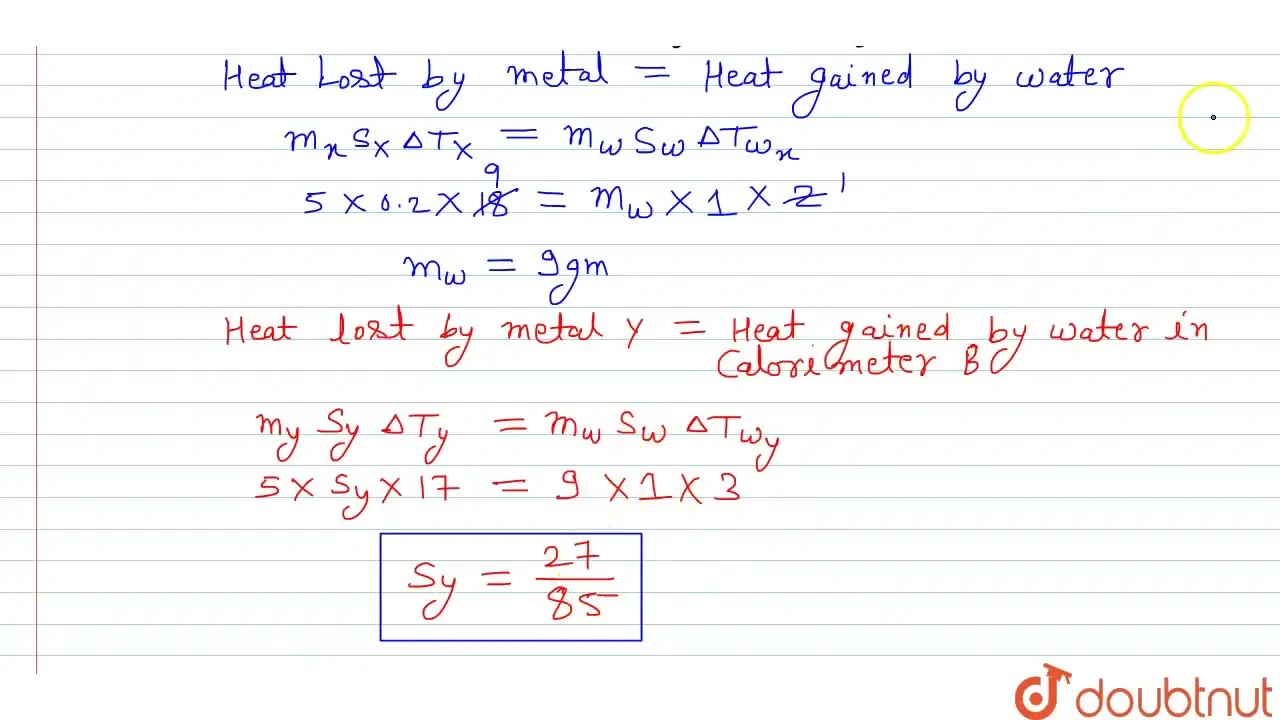
Two identical calorimeters A and B contain an equal quantity of water

Solved 3. A block of copper of unknown mass has an initial

OpenStax College Physics, Chapter 14, Problem 13 (Problems & Exercises)

What will be the equilibrium temperature when a 245-g block

Specific Heat Capacity
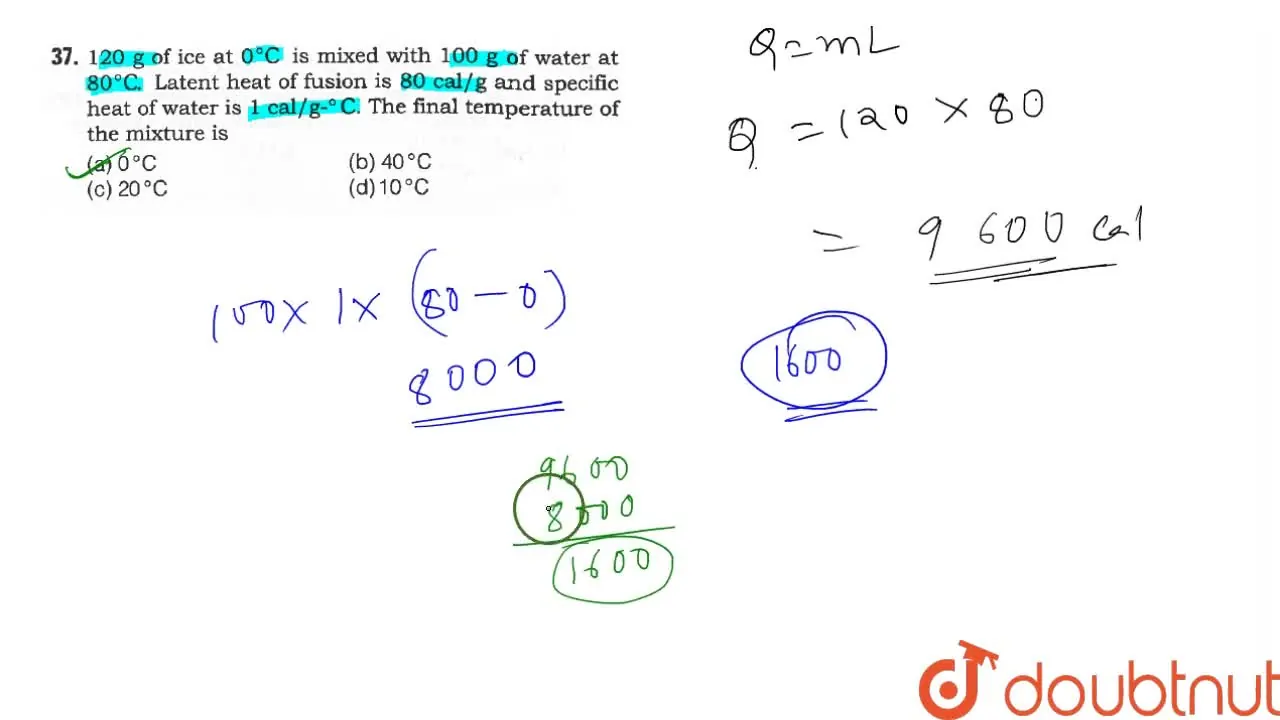
120 g of ice at 0^(@)C is mixed with 100 g of water at 80^(@)C. Latent

What will be the equilibrium temperature when a 245-g block

Answered: 1. What is the specific heat of a…
Ammonia - Wikipedia
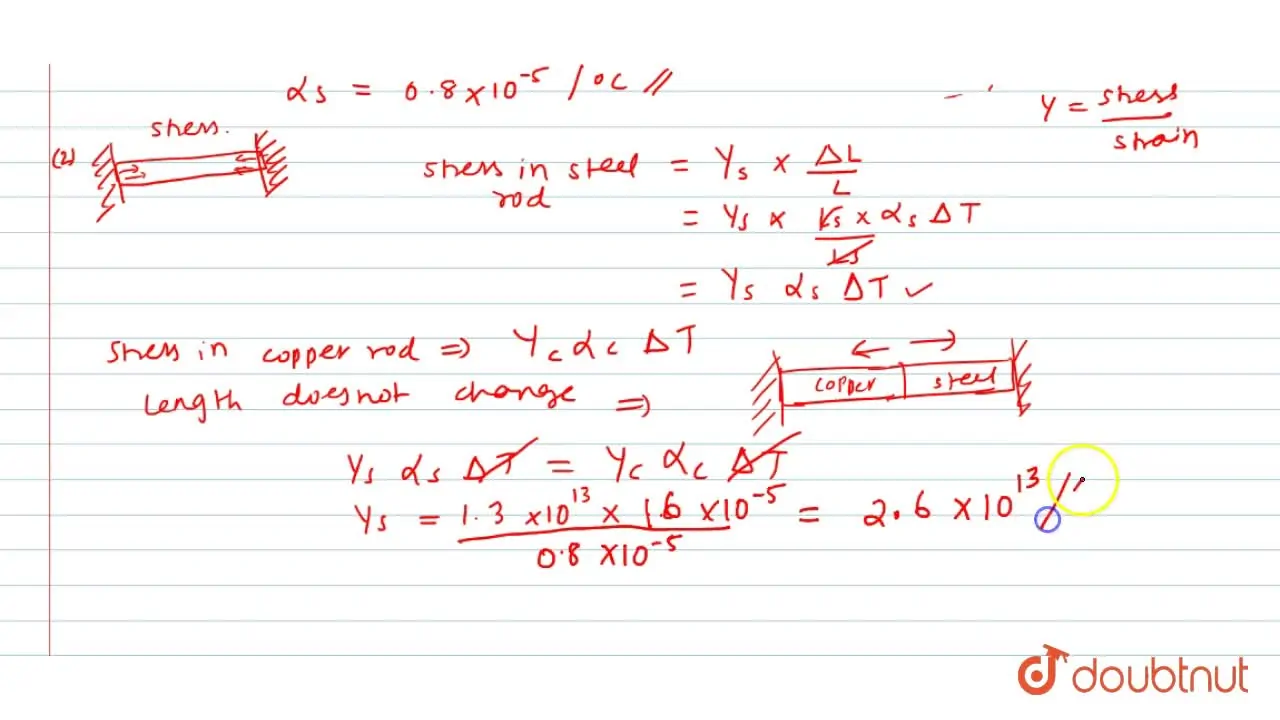
Two rods of equal cross sections, one of copper and the other of steel