32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting

20.0 kg of H2 and 32 kg of O2 are reacted to produce H2O.the

80g of H2 is reacted with 80g of O2 to form water; what are the
If 50 grams of each reactant is available in reaction C +O2, which

SOLVED: Which is the limiting reactant when 5.00 g of H2 and 10.0

2g of hydrogen combine with 16g of oxygen to form water and with

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?

3 g of H2 react with 29 g of O2
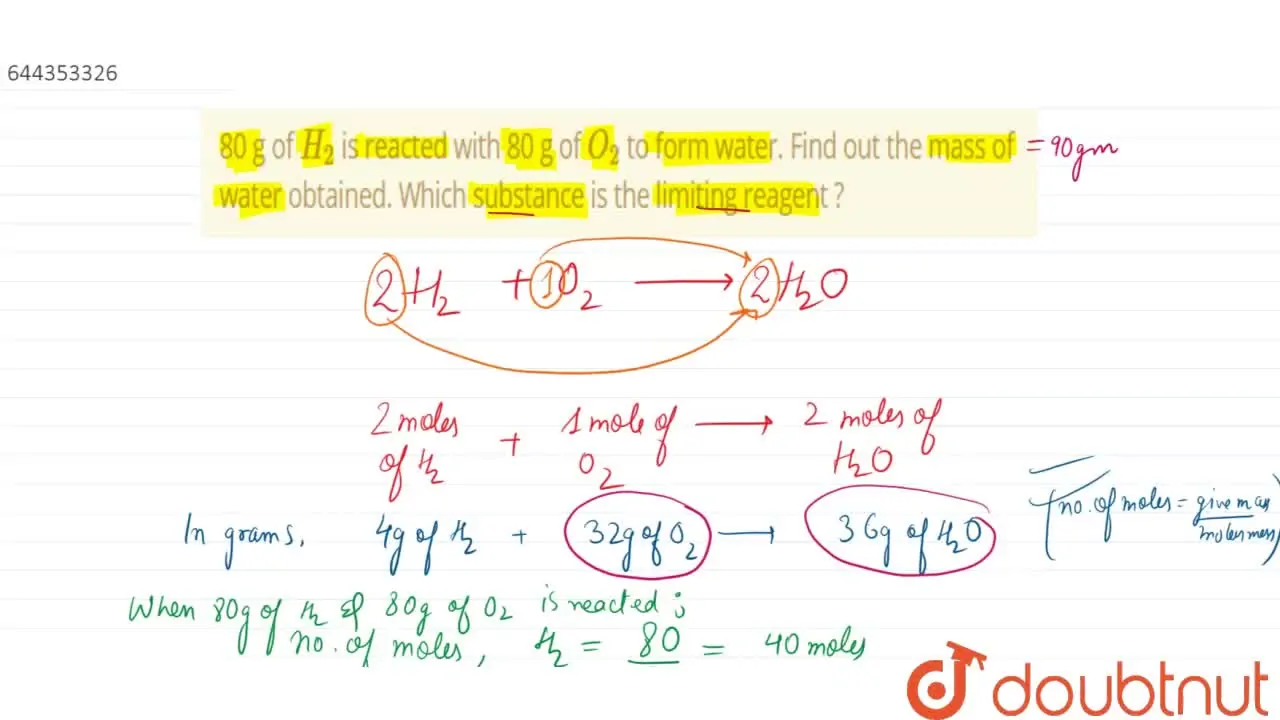
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the
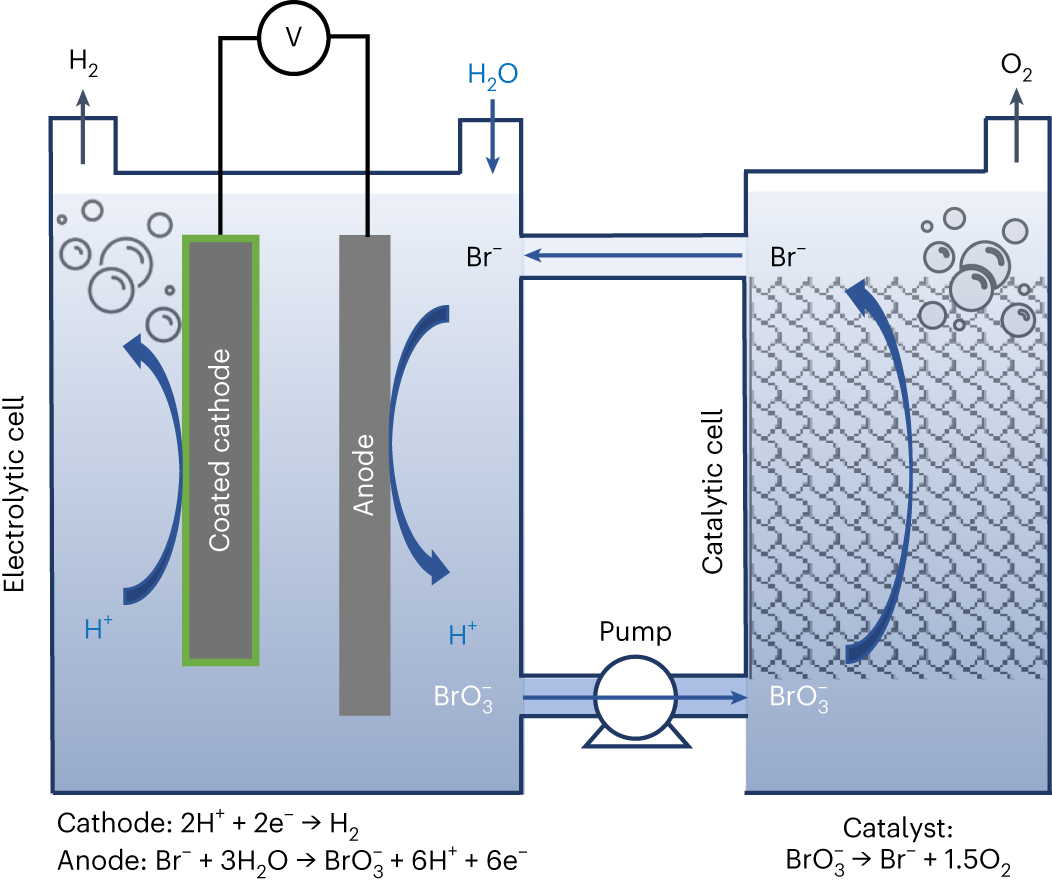
Electrochemical and chemical cycle for high-efficiency decoupled